A genetic mouse model of severe iron deficiency anemia reveals tissue-specific transcriptional stress responses and cardiac remodeling
- PMID: 31416832
- PMCID: PMC6791316
- DOI: 10.1074/jbc.RA119.009578
A genetic mouse model of severe iron deficiency anemia reveals tissue-specific transcriptional stress responses and cardiac remodeling
Abstract
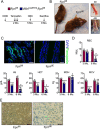
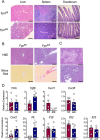
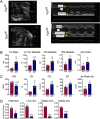
Iron is a micronutrient fundamental for life. Iron homeostasis in mammals requires sustained postnatal intestinal iron absorption that maintains intracellular iron concentrations for central and systemic metabolism as well as for erythropoiesis and oxygen transport. More than 1 billion people worldwide suffer from iron deficiency anemia (IDA), a state of systemic iron insufficiency that limits the production of red blood cells and leads to tissue hypoxia and intracellular iron stress. Despite this tremendous public health concern, very few genetic models of IDA are available to study its progression. Here we developed and characterized a novel genetic mouse model of IDA. We found that tamoxifen-inducible deletion of the mammalian iron exporter ferroportin exclusively in intestinal epithelial cells leads to loss of intestinal iron absorption. Ferroportin ablation yielded a robust phenotype of progressive IDA that develops in as little as 3 months following disruption of intestinal iron absorption. We noted that, at end-stage IDA, tissue-specific transcriptional stress responses occur in which the heart shows little to no hypoxic and iron stress compared with other peripheral organs. However, morphometric and echocardiographic analysis revealed massive cardiac hypertrophy and chamber dilation, albeit with increased cardiac output at very low basal heart rates. We propose that our intestine-specific ferroportin knockout mouse model of end-stage IDA could be used in future studies to investigate IDA progression and cell-specific responses to hypoxic and iron stress.
Keywords: animal model; hypoxia; hypoxia-inducible factor (HIF); iron; iron metabolism.
© 2019 Schwartz et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

