Genetic behavioral screen identifies an orphan anti-opioid system
- PMID: 31416932
- PMCID: PMC7074901
- DOI: 10.1126/science.aau2078
Genetic behavioral screen identifies an orphan anti-opioid system
Abstract
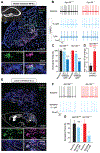
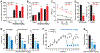
Opioids target the μ-opioid receptor (MOR) to produce unrivaled pain management, but their addictive properties can lead to severe abuse. We developed a whole-animal behavioral platform for unbiased discovery of genes influencing opioid responsiveness. Using forward genetics in Caenorhabditis elegans, we identified a conserved orphan receptor, GPR139, with anti-opioid activity. GPR139 is coexpressed with MOR in opioid-sensitive brain circuits, binds to MOR, and inhibits signaling to heterotrimeric guanine nucleotide-binding proteins (G proteins). Deletion of GPR139 in mice enhanced opioid-induced inhibition of neuronal firing to modulate morphine-induced analgesia, reward, and withdrawal. Thus, GPR139 could be a useful target for increasing opioid safety. These results also demonstrate the potential of C. elegans as a scalable platform for genetic discovery of G protein-coupled receptor signaling principles.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

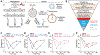

Comment in
-
Countering opioid side effects.Science. 2019 Sep 20;365(6459):1246-1247. doi: 10.1126/science.aay9345. Science. 2019. PMID: 31604226 No abstract available.
-
An Anti-Opioid System, Courtesy of a Worm Model.N Engl J Med. 2019 Nov 21;381(21):2067-2069. doi: 10.1056/NEJMcibr1911069. N Engl J Med. 2019. PMID: 31747732 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

