Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer
- PMID: 31417189
- PMCID: PMC6889319
- DOI: 10.1038/s41416-019-0542-2
Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer
Erratum in
-
Correction: Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer.Br J Cancer. 2020 Mar;122(7):1118. doi: 10.1038/s41416-019-0710-4. Br J Cancer. 2020. PMID: 31937927 Free PMC article.
Abstract
Background: Tumours rapidly ferment glucose to lactic acid even in the presence of oxygen, and coupling high glycolysis with poor perfusion leads to extracellular acidification. We hypothesise that acidity, independent from lactate, can augment the pro-tumour phenotype of macrophages.
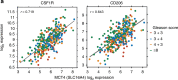
Methods: We analysed publicly available data of human prostate cancer for linear correlation between macrophage markers and glycolysis genes. We used zwitterionic buffers to adjust the pH in series of in vitro experiments. We then utilised subcutaneous and transgenic tumour models developed in C57BL/6 mice as well as computer simulations to correlate tumour progression with macrophage infiltration and to delineate role of acidity.
Results: Activating macrophages at pH 6.8 in vitro enhanced an IL-4-driven phenotype as measured by gene expression, cytokine profiling, and functional assays. These results were recapitulated in vivo wherein neutralising intratumoural acidity reduced the pro-tumour phenotype of macrophages, while also decreasing tumour incidence and invasion in the TRAMP model of prostate cancer. These results were recapitulated using an in silico mathematical model that simulate macrophage responses to environmental signals. By turning off acid-induced cellular responses, our in silico mathematical modelling shows that acid-resistant macrophages can limit tumour progression.
Conclusions: This study suggests that tumour acidity contributes to prostate carcinogenesis by altering the state of macrophage activation.
Conflict of interest statement
R. Gillies has a sponsored research support from Helix Biopharma. The other authors declare no competing interests.
Figures




Comment in
-
Prostate tumour pH affects macrophage function.Nat Rev Urol. 2019 Oct;16(10):566-567. doi: 10.1038/s41585-019-0236-9. Nat Rev Urol. 2019. PMID: 31520082 No abstract available.
References
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

