Antitumor and antibacterial properties of virally encoded cationic sequences
- PMID: 31417238
- PMCID: PMC6599856
- DOI: 10.2147/BTT.S201287
Antitumor and antibacterial properties of virally encoded cationic sequences
Abstract
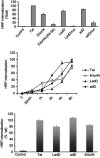
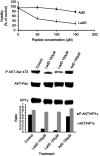
Objective: The objective of this study was to test our Viral Quinta Columna Strategy (VQCS), a new biological hypothesis predicting that specific multifunctional virally encoded cationic domains may have the capacity to penetrate human cells and interact with PP2A proteins to deregulate important human intracellular pathways, and may display LL37 cathelicidin-like antagonistic effects against multiple pathogens such as bacteria or viruses. Methods: We comparatively analyzed the host defense properties of adenodiaphorins and of some specific cationic sequences encoded by different viruses using two distinct biological models: U87G, a well-characterized cell tumor model; and a group B Streptococcus agalactiae NEM316 ΔdltA, highly sensitive to LL37 cathelicidin. Results: We found that the adenovirus type 2 E4orf4 is a cell-permeable protein containing a new E4orf464-95 protein transduction domain, named large adenodiaphorin or LadD64-95. Interestingly, the host defense LL37 peptide is the unique cathelicidin in humans. In this context, we also demonstrated that similarly to LL37 LadD64-95, several virally encoded cationic sequences including the C-terminus HIV-1 89.6 Vpr77-92, shorter adenodiaphorins AdD67-84/AdD/69-84/AdD69-83, as well as HIV-2 Tat67-90 and JC polyomavirus small t115-134, displayed similar toxicity against Gram-positive S. agalactiae NEM316 ΔdltA strain. Finally, LadD64-95, adenodiaphorin AdD67-84, AdD69-84, and LL37 and LL17-32 cathelicidin peptides also inhibited the survival of human U87G glioblastoma cells. Conclusion: In this study, we demonstrated that specific cationic sequences encoded by four different viruses displayed antibacterial activities against S. agalactiae NEM316 ΔdltA strain. In addition, HIV-1 Vpr71-92 and adenovirus 2 E4orf464-95, two cationic penetrating sequences that bind PP2A, inhibited the survival of U87G glioblastoma cells. These results illustrate the host defense properties of virally encoded sequences and could represent an initial step for future complete validation of the VQCS hypothesis.
Keywords: PP2A; bacteria; cancer; cationic sequences; viruses.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures

References
-
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. - PubMed
-
- Pooga M, Langel U. Synthesis of cell-penetrating peptides for cargo delivery. Methods Mol Biol. 2005;298:77–89. - PubMed
LinkOut - more resources
Full Text Sources

