Macrophages as a Source and Recipient of Wnt Signals
- PMID: 31417574
- PMCID: PMC6685136
- DOI: 10.3389/fimmu.2019.01813
Macrophages as a Source and Recipient of Wnt Signals
Abstract
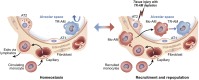
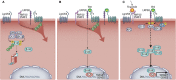
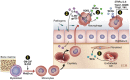
Macrophages are often viewed through the lens of their core functions, but recent transcriptomic studies reveal them to be largely distinct across tissue types. While these differences appear to be shaped by their local environment, the key signals that drive these transcriptional differences remain unclear. Since Wnt signaling plays established roles in cell fate decisions, and tissue patterning during development and tissue repair after injury, we consider evidence that Wnt signals both target and are affected by macrophage functions. We propose that the Wnt gradients present in developing and adult tissues effectively shape macrophage fates and phenotypes. We also highlight evidence that macrophages, through an ability to dispatch Wnt signals, may couple tissue debridement and matrix remodeling with stem cell activation and tissue repair.
Keywords: Wnt signaling; beta catenin; immunity; macrophages; monocytes.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

