Lowering Lipophilicity by Adding Carbon: AzaSpiroHeptanes, a log D Lowering Twist
- PMID: 31417667
- PMCID: PMC6693472
- DOI: 10.1021/acsmedchemlett.9b00248
Lowering Lipophilicity by Adding Carbon: AzaSpiroHeptanes, a log D Lowering Twist
Abstract
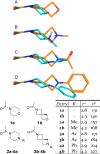
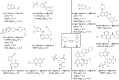
We have conducted an analysis of azaspiro[3.3]heptanes used as replacements for morpholines, piperidines, and piperazines in a medicinal chemistry context. In most cases, introducing a spirocyclic center lowered the measured logD 7.4 of the corresponding molecules by as much as -1.0 relative to the more usual heterocycle. This may seem counterintuitive, as the net change in the molecule is the addition of a single carbon atom, but it may be rationalized in terms of increased basicity. An exception to this was found with N-linked 2-azaspiro[3.3]heptane, where logD 7.4 increased by as much as +0.5, consistent with the addition of carbon. During our investigation, we also concluded that azaspiro[3.3]heptanes are most likely not suitable bioisosteres for morpholines, piperidines, and piperazines, when not used as terminal groups, due to significant changes in their geometry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
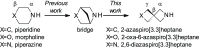

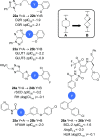
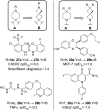

References
-
- Wuitschik G.; Rogers-Evans M.; Buckl A.; Bernasconi M.; Marki M.; Godel T.; Fischer H.; Wagner B.; Parrilla I.; Schuler F.; Schneider J.; Alker A.; Schweizer W. B.; Muller K.; Carreira E. M. Spirocyclic oxetanes: synthesis and properties. Angew. Chem., Int. Ed. 2008, 47, 4512–4515. 10.1002/anie.200800450. - DOI - PubMed
-
- Alelyunas Y. W.; Pelosi-Kilby L.; Turcotte P.; Kary M. B.; Spreen R. C. A high throughput dried DMSO LogD lipophilicity measurement based on 96-well shake-flask and atmospheric pressure photoionization mass spectrometry detection. J. Chromatogr. A 2010, 1217, 1950–1955. 10.1016/j.chroma.2010.01.071. - DOI - PubMed
-
- Scott J. S.; Degorce S. L.; Anjum R.; Culshaw J.; Davies R. M. D.; Davies N. L.; Dillman K. S.; Dowling J. E.; Drew L.; Ferguson A. D.; Groombridge S. D.; Halsall C. T.; Hudson J. A.; Lamont S.; Lindsay N. A.; Marden S. K.; Mayo M. F.; Pease J. E.; Perkins D. R.; Pink J. H.; Robb G. R.; Rosen A.; Shen M.; McWhirter C.; Wu D. Discovery and optimization of pyrrolopyrimidine inhibitors of Interleukin-1 Receptor Associated Kinase 4 (IRAK4) for the treatment of mutant MYD88L265P diffuse large B-cell lymphoma. J. Med. Chem. 2017, 60, 10071–10091. 10.1021/acs.jmedchem.7b01290. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

