(E)-6,6'-(Diazene-1,2-di-yl)bis-(1,10-phenanthrolin-5-ol) tri-chloro-methane disolvate: a superconjugated ligand
- PMID: 31417796
- PMCID: PMC6690457
- DOI: 10.1107/S205698901900954X
(E)-6,6'-(Diazene-1,2-di-yl)bis-(1,10-phenanthrolin-5-ol) tri-chloro-methane disolvate: a superconjugated ligand
Abstract
Phenanthroline ligands are important metal-binding mol-ecules which have been extensively researched for applications in both material science and medicinal chemistry. Azo-benzene and its derivatives have received significant attention because of their ability to be reversibly switched between the E and Z forms and so could have applications in optical memory and logic devices or as mol-ecular machines. Herein we report the formation and crystal structure of a highly unusual novel diazo-diphenanthroline compound, C24H14N6O2·2CHCl3.
Keywords: crystal structure; diazo; phenanthroline; superconjugated ligand.
Figures
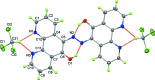



References
-
- Bencini, A. & Lippolis, V. (2010). Coord. Chem. Rev. 254, 2096–2180.
-
- Bhutani, H., Singh, S., Vir, S., Bhutani, K. K., Kumar, R., Chakraborti, A. K. & Jindal, K. C. (2007). J. Pharm. Biomed. Anal. 43, 1213–1220. - PubMed
-
- Bruker (2010). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Casey, M. T., McCann, M., Devereux, M., Curran, M., Cardin, C., Convery, M., Quillet, V. & Harding, C. (1994). J. Chem. Soc. Chem. Commun. pp. 2643–2645.
LinkOut - more resources
Full Text Sources
Miscellaneous
