Cellulose Nano-Films as Bio-Interfaces
- PMID: 31417896
- PMCID: PMC6682661
- DOI: 10.3389/fchem.2019.00535
Cellulose Nano-Films as Bio-Interfaces
Abstract
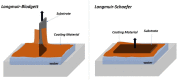
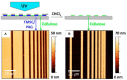
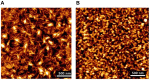
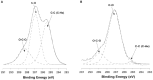
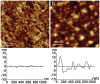
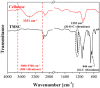
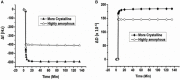
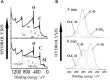
Cellulose, the most abundant polymer on earth, has enormous potential in developing bio-friendly, and sustainable technological products. In particular, cellulose films of nanoscale thickness (1-100 nm) are transparent, smooth (roughness <1 nm), and provide a large surface area interface for biomolecules immobilization and interactions. These attractive film properties create many possibilities for both fundamental studies and applications, especially in the biomedical field. The three liable-OH groups on the monomeric unit of the cellulose chain provide schemes to chemically modify the cellulose interface and engineer its properties. Here, the cellulose thin film serves as a substrate for biomolecules interactions and acts as a support for bio-diagnostics. This review focuses on the challenges and opportunities provided by engineering cellulose thin films for controlling biomolecules interactions. The first part reviews the methods for preparing cellulose thin films. These are by dispersing or dissolving pure cellulose or cellulose derivatives in a solvent to coat a substrate using the spin coating, Langmuir-Blodgett, or Langmuir-Schaefer method. It is shown how different cellulose sources, preparation, and coating methods and substrate surface pre-treatment affect the film thickness, roughness, morphology, crystallinity, swelling in water, and homogeneity. The second part analyses the bio-macromolecules interactions with the cellulose thin film interfaces. Biomolecules, such as antibodies and enzymes, are adsorbed at the cellulose-liquid interface, and analyzed dry and wet. This highlights the effect of film surface morphology, thickness, crystallinity, water intake capacity, and surface pre-treatment on biomolecule adsorption, conformation, coverage, longevity, and activity. Advance characterization of cellulose thin film interface morphology and adsorbed biomolecules interactions are next reviewed. X-ray and neutron scattering/reflectivity combined with atomic force microscopy (AFM), quartz crystal microbalance (QCM), microscopy, and ellipsometer allow visualizing, and quantifying the structural morphology of cellulose-biomolecule interphase and the respective biomolecules conformations, kinetics, and sorption mechanisms. This review provides a novel insight on the advantages and challenges of engineering cellulose thin films for biomedical applications. This is to foster the exploration at the molecular level of the interaction mechanisms between a cellulose interface and adsorbed biomolecules with respect to adsorbed molecules morphology, surface coverage, and quantity. This knowledge is to engineer a novel generation of efficient and functional biomedical devices.
Keywords: biomolecule; cellulose; characterization; diagnostics; interface; thin film.
Figures






References
-
- Ahola S., Myllytie P., Österberg M., Teerinen T., Laine J. (2008). Effect of polymer adsroption on cellulose nanofibril water binding capacity and aggregation. Bioresources 3, 1315–1328.
-
- Arcot L. R., Chen X., Wenchao X., Johansson L. S., Rojas O. J. (2015). Paper-based plasmon-enhanced protein sensing by controlled nucleation of silver nanoparticles on cellulose. Cellulose 22, 4027–4034. 10.1007/s10570-015-0783-z - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

