A review of microsampling techniques and their social impact
- PMID: 31418068
- PMCID: PMC6695349
- DOI: 10.1007/s10544-019-0412-y
A review of microsampling techniques and their social impact
Abstract
Conventional skin and blood sampling techniques for disease diagnosis, though effective, are often highly invasive and some even suffer from variations in analysis. With the improvements in molecular detection, the amount of starting sample quantity needed has significantly reduced in some diagnostic procedures, and this has led to an increased interest in microsampling techniques for disease biomarker detection. The miniaturization of sampling platforms driven by microsampling has the potential to shift disease diagnosis and monitoring closer to the point of care. The faster turnaround time for actionable results has improved patient care. The variations in sample quantification and analysis remain a challenge in the microsampling field. The future of microsampling looks promising. Emerging techniques are being clinically tested and monitored by regulatory bodies. This process is leading to safer and more reliable diagnostic platforms. This review discusses the advantages and disadvantages of current skin and blood microsampling techniques.
Keywords: Blood sampling; Microneedle; Microsampling; Minimally invasive; Point-of-care device; Skin biopsy.
Figures




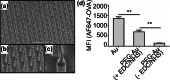
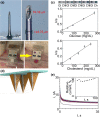

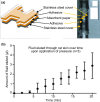
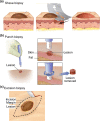






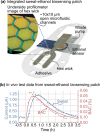


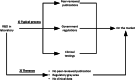
References
-
- Ackerman N, et al. Glucose monitoring via reverse iontophoresis. American Chemical Society, Polymer Preprints, Division of Polym. Chem. 1999;40:303–304.
-
- Andrews E, et al. Concurrent human papillomavirus–associated tonsillar carcinoma in 2 couples. J. Infect. Dis. 2009;200:882–887. - PubMed
-
- Asha SE, et al. Impact from point-of-care devices on emergency department patient processing times compared with central laboratory testing of blood samples: A randomised controlled trial and cost-effectiveness analysis. Emerg. Med. J. 2014;31:714–719. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

