Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017
- PMID: 31418085
- PMCID: PMC7485122
- DOI: 10.1007/s00330-019-06376-5
Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017
Erratum in
-
Correction to: Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017.Eur Radiol. 2021 Jun;31(6):4400-4401. doi: 10.1007/s00330-020-07444-x. Eur Radiol. 2021. PMID: 33226455 No abstract available.
Abstract
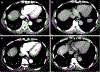
Objective: To investigate the performance of Liver Imaging Reporting and Data System (LI-RADS) v2017 treatment response algorithm for predicting hepatocellular carcinoma (HCC) viability after locoregional therapy (LRT) using the liver explant as reference.
Methods: One hundred fourteen patients with 206 HCCs who underwent liver transplantation (LT) after LRT for HCCs were included in this retrospective study. Two radiologists independently evaluated tumor viability using the LI-RADS and modified RECIST (mRECIST) with CT and MRI, respectively. The sensitivity and specificity of arterial phase hyperenhancement (APHE) and LR-TR viable criteria (any of three findings: APHE, washout, and enhancement pattern similar to pretreatment imaging) were compared using logistic regression. Receiver operating characteristics (ROC) analysis was used to compare the diagnostic performance between LI-RADS and mRECIST and between CT and MRI.
Results: The sensitivity and specificity for diagnosing viable tumor were not significantly different between APHE alone and LR-TR viable criteria on CT (p = 0.054 and p = 0.317) and MRI (p = 0.093 and p = 0.603). On CT, the area under the ROC curve (AUC) of LI-RADS was significantly higher than that of mRECIST (0.733 vs. 0.657, p < 0.001). On MRI, there was no significant difference in AUCs between LI-RADS and mRECIST (0.802 vs. 0.791, p = 0.500). Intra-individual comparison of CT and MRI showed comparable AUCs using LI-RADS (0.783 vs. 0.795, p = 0.776).
Conclusions: LI-RADS v2017 treatment response algorithm showed better diagnostic performance than mRECIST on CT. With LI-RADS, CT and MRI were comparable to diagnose tumor viability of HCC after LRT.
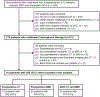
Key points: • Using Liver Imaging Reporting and Data System (LI-RADS) v2017 treatment response algorithm, the viability of hepatocellular carcinoma (HCC) after locoregional therapy (LRT) can be accurately diagnosed. • LI-RADS v2017 treatment response algorithm is superior to modified Response Evaluation Criteria in Solid Tumors for evaluating HCC viability using CT. • Either CT or MRI can be performed to assess tumor viability after LRT using LI-RADS v2017 treatment response algorithm.
Keywords: Hepatocellular carcinoma; Liver transplantation; Magnetic resonance imaging; Multidetector computed tomography; Therapeutic chemoembolization.
Conflict of interest statement
Conflict of interest:
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures


Similar articles
-
LI-RADS Nonradiation Treatment Response Algorithm Version 2024: Diagnostic Performance and Impact of Ancillary Features.AJR Am J Roentgenol. 2025 Feb;224(2):e2432035. doi: 10.2214/AJR.24.32035. Epub 2024 Nov 13. AJR Am J Roentgenol. 2025. PMID: 39535775
-
Evaluation of LI-RADS Version 2018 Treatment Response Algorithm for Hepatocellular Carcinoma in Liver Transplant Candidates: Intraindividual Comparison between CT and Hepatobiliary Agent-enhanced MRI.Radiology. 2021 May;299(2):336-345. doi: 10.1148/radiol.2021203537. Epub 2021 Mar 2. Radiology. 2021. PMID: 33650901
-
LI-RADS version 2018 treatment response algorithm on extracellular contrast-enhanced MRI in patients treated with transarterial chemoembolization for hepatocellular carcinoma: diagnostic performance and the added value of ancillary features.Abdom Radiol (NY). 2024 Sep;49(9):3045-3055. doi: 10.1007/s00261-024-04275-y. Epub 2024 Apr 11. Abdom Radiol (NY). 2024. PMID: 38605217
-
Diagnostic Performance of CT/MRI Liver Imaging Reporting and Data System v2017 for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis.Liver Int. 2020 Jun;40(6):1488-1497. doi: 10.1111/liv.14424. Epub 2020 Mar 19. Liver Int. 2020. PMID: 32145134
-
LI-RADS treatment response algorithm for detecting incomplete necrosis in hepatocellular carcinoma after locoregional treatment: a systematic review and meta-analysis using individual patient data.Abdom Radiol (NY). 2021 Aug;46(8):3717-3728. doi: 10.1007/s00261-021-03122-8. Epub 2021 May 23. Abdom Radiol (NY). 2021. PMID: 34027566 Free PMC article.
Cited by
-
Moving Away from Uncertainty: A Potential Role for Ancillary Features in LI-RADS Treatment Response.Radiology. 2020 Sep;296(3):562-563. doi: 10.1148/radiol.2020202637. Epub 2020 Jul 21. Radiology. 2020. PMID: 32697166 Free PMC article. No abstract available.
-
CT volume of enhancement of disease (VED) can predict the early response to treatment and overall survival in patients with advanced HCC treated with sorafenib.Eur Radiol. 2021 Mar;31(3):1608-1619. doi: 10.1007/s00330-020-07171-3. Epub 2020 Aug 22. Eur Radiol. 2021. PMID: 32827266 Free PMC article.
-
LI-RADS Version 2018 Treatment Response Algorithm: Diagnostic Performance after Transarterial Radioembolization for Hepatocellular Carcinoma.Korean J Radiol. 2021 Aug;22(8):1279-1288. doi: 10.3348/kjr.2020.1159. Epub 2021 May 4. Korean J Radiol. 2021. PMID: 33987991 Free PMC article.
-
LI-RADS Treatment Response versus Modified RECIST for Diagnosing Viable Hepatocellular Carcinoma after Locoregional Therapy: A Systematic Review and Meta-Analysis of Comparative Studies.Taehan Yongsang Uihakhoe Chi. 2022 Mar;83(2):331-343. doi: 10.3348/jksr.2021.0173. Epub 2022 Feb 25. Taehan Yongsang Uihakhoe Chi. 2022. PMID: 36237934 Free PMC article.
-
Hepatocellular carcinoma Liver Imaging Reporting and Data Systems treatment response assessment: Lessons learned and future directions.World J Hepatol. 2020 Oct 27;12(10):738-753. doi: 10.4254/wjh.v12.i10.738. World J Hepatol. 2020. PMID: 33200013 Free PMC article. Review.
References
-
- Yao FY, Fidelman N (2016) Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology 63:1014–1025 - PubMed
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214 - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216 - PubMed
-
- Bruix J, Sherman M, Llovet JM et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430 - PubMed
-
- Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

