Metabolic implications of organelle-mitochondria communication
- PMID: 31418169
- PMCID: PMC6726909
- DOI: 10.15252/embr.201947928
Metabolic implications of organelle-mitochondria communication
Abstract
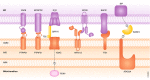
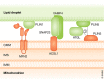
Cellular organelles are not static but show dynamism-a property that is likely relevant for their function. In addition, they interact with other organelles in a highly dynamic manner. In this review, we analyze the proteins involved in the interaction between mitochondria and other cellular organelles, especially the endoplasmic reticulum, lipid droplets, and lysosomes. Recent results indicate that, on one hand, metabolic alterations perturb the interaction between mitochondria and other organelles, and, on the other hand, that deficiency in proteins involved in the tethering between mitochondria and the ER or in specific functions of the interaction leads to metabolic alterations in a variety of tissues. The interaction between organelles is an emerging field that will permit to identify key proteins, to delineate novel modulation pathways, and to elucidate their implications in human disease.
Keywords: contact sites; diabetes; endoplasmic reticulum; insulin resistance; lipid droplets.
© 2019 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
The authors declare that they have no conflict of interest.
Figures




References
-
- Dolman NJ, Gerasimenko JV, Gerasimenko OV, Voronina SG, Petersen OH, Tepikin AV (2005) Stable Golgi‐mitochondria complexes and formation of Golgi Ca2+ gradients in pancreatic acinar cells. J Biol Chem 280: 15794–15799 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

