Polyampholytes as Emerging Macromolecular Cryoprotectants
- PMID: 31418266
- PMCID: PMC6960013
- DOI: 10.1021/acs.biomac.9b01053
Polyampholytes as Emerging Macromolecular Cryoprotectants
Abstract
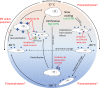
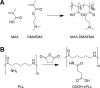
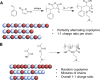
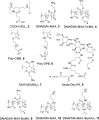
Cellular cryopreservation is a platform technology which underpins cell biology, biochemistry, biomaterials, diagnostics, and the cold chain for emerging cell-based therapies. This technique relies on effective methods for banking and shipping to avoid the need for continuous cell culture. The most common method to achieve cryopreservation is to use large volumes of organic solvent cryoprotective agents which can promote either a vitreous (ice free) phase or dehydrate and protect the cells. These methods are very successful but are not perfect: not all cell types can be cryopreserved and recovered, and the cells do not always retain their phenotype and function post-thaw. This Perspective will introduce polyampholytes as emerging macromolecular cryoprotective agents and demonstrate they have the potential to impact a range of fields from cell-based therapies to basic cell biology and may be able to improve, or replace, current solvent-based cryoprotective agents. Polyampholytes have been shown to be remarkable (mammalian cell) cryopreservation enhancers, but their mechanism of action is unclear, which may include membrane protection, solvent replacement, or a yet unknown protective mechanism, but it seems the modulation of ice growth (recrystallization) may only play a minor role in their function, unlike other macromolecular cryoprotectants. This Perspective will discuss their synthesis and summarize the state-of-the-art, including hypotheses of how they function, to introduce this exciting area of biomacromolecular science.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.I.G., T.L.B. and C.S. are named inventors on a patent application using materials related to those mentioned in this Perspective.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

