Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function
- PMID: 31418947
- PMCID: PMC6869122
- DOI: 10.1111/cpr.12673
Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function
Abstract
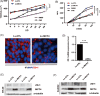
Objective: Inflammatory bowel disease (IBD) is a disorder intestinal inflammation and impaired barrier function, associated with increased epithelial expression of monocarboxylate transporter 4 (MCT4). However, the specific non-metabolic function and clinical relevance of MCT4 in IBD remain to be fully elucidated.
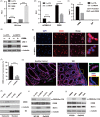
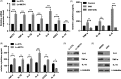
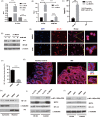
Methods: Lentivirus-mediated overexpression of MCT4 was used to assess the role of MCT4 in transcriptionally regulating ZO-1 and IL-6 expression by luciferase assays, WB and ChIP. IP was used to analyse the effect of MCT4 on the interaction NF-κB-CBP or CREB-CBP, and these MCT4-mediated effects were confirmed in vivo assay.
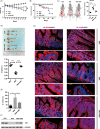
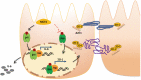
Results: We showed that ectopic expression of MCT4 inhibited ZO-1 expression, while increased pro-inflammatory factors expression, leading to destroy intestinal epithelial barrier function in vitro and in vivo. Mechanistically, MCT4 contributed NF-κB p65 nuclear translocation and increased the binding of NF-κB p65 to the promoter of IL-6, which is attributed to MCT4 enhanced NF-κB-CBP interaction and dissolved CREB-CBP complex, resulting in reduction of CREB activity and CREB-mediated ZO-1 expression. In addition, treatment of experimental colitis with MCT4 inhibitor α-cyano-4-hydroxycinnamate (CHC) ameliorated mucosal intestinal barrier function, which was due to attenuation of pro-inflammation factors expression and enhancement of ZO-1 expression.
Conclusion: These findings suggested a novel role of MCT4 in controlling development of IBD and provided evidence for potential targets of IBD.
Keywords: CREB; IL-6; ZO-1; inflammatory bowel disease; monocarboxylate transporter 4; nuclear factor-κB.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declared that they have no conflict of interests.
Figures


References
-
- Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka‐Walus A. Quality of life in inflammatory bowel disease: a systematic review and meta‐analyses‐part I. Inflamm Bowel Dis. 2018;24(4):742‐751. - PubMed
-
- Taylor K, Scruggs PW, Balemba OB, Wiest MM, Vella CA. Associations between physical activity, resilience, and quality of life in people with inflammatory bowel disease. Eur J Appl Physiol. 2018;118(4):829‐836. - PubMed
-
- Holmberg F, Pedersen J, Jorgensen P, Soendergaard C, Jensen KB, Nielsen OH. Intestinal barrier integrity and inflammatory bowel disease: Stem cell‐based approaches to regenerate the barrier. J Tissue Eng Regen Med. 2018;12(4):923‐935. - PubMed
-
- Henderson P, van Limbergen JE, Schwarze J, Wilson DC. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):382‐395. - PubMed
MeSH terms
Substances
Grants and funding
- 81770552/National Natural Science Foundation of China
- 81860101/National Natural Science Foundation of China
- 2017A030313838/Natural Science Foundation of Guangdong Province
- 2018A0303130175/Natural Science Foundation of Guangdong Province
- A2018395/Medical Science and Technology Foundation of Guangdong
- A2019534/Medical Science and Technology Foundation of Guangdong
- 201804010148, 907171491046/Guangzhou Municipal Science and Technology Project
- 201904010485/Guangzhou Municipal Science and Technology Project
- 20191A010024/Guangzhou Municipal Health and Family Planning Commission
- 2018M643043/China Postdoctoral Science Foundation
- 5001-3001075/Postdoctoral Research Funding of Guangzhou Women and Children's Medical Center
- IP-2016-005/Guangzhou Institute of Pediatrics/Guangzhou Women and Children's Medical Center
- IP-2018-009/Guangzhou Institute of Pediatrics/Guangzhou Women and Children's Medical Center
- 201704020223/Guangzhou Women and Children's Medical Center and Sun Yat-Sen University
- 2819QN372/Natural Science Foundation of Hainan Province
LinkOut - more resources
Full Text Sources
Other Literature Sources

