Flavylium Salts: A Blooming Core for Bioinspired Ionic Liquid Crystals
- PMID: 31418972
- PMCID: PMC6856849
- DOI: 10.1002/chem.201901975
Flavylium Salts: A Blooming Core for Bioinspired Ionic Liquid Crystals
Abstract
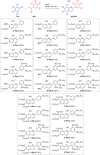
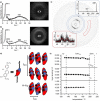
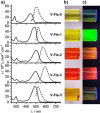
Thermotropic ionic liquid crystals based on the flavylium scaffold have been synthesized and studied for their structure-properties relationship for the first time. The mesogens were probed by differential scanning calorimetry (DSC), polarizing optical microscopy (POM), and X-ray diffraction (XRD). Low numbers of alkoxy side chains resulted in smectic (SmA) and lamello-columnar (LamCol ) phases, whereas higher substituted flavylium salts showed Colro as well as ordered and disordered columnar (Colho , Colhd ) mesophases. Mesophase width ranged from 13 K to 220 K, giving access to room temperature liquid crystals. The optical properties of the synthesized compounds were probed towards absorption and emission properties. Strong absorption with maxima between 444 and 507 nm was observed, and some chromophores were highly emissive with quantum yields up to 99 %. Ultimately, mesogenic and dye properties were examined by temperature-dependent emissive experiments in the solid state.
Keywords: UV/Vis spectroscopy; X-ray diffraction; fluorescence; ionic liquid crystals; self-assembly.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














Similar articles
-
Chasing Self-Assembly of Thioether-Substituted Flavylium Salts in Solution and Bulk State.Chemphyschem. 2022 Jul 5;23(13):e202200154. doi: 10.1002/cphc.202200154. Epub 2022 May 17. Chemphyschem. 2022. PMID: 35446455 Free PMC article.
-
Diketonylpyridinium Cations as a Support of New Ionic Liquid Crystals and Ion-Conductive Materials: Analysis of Counter-Ion Effects.Materials (Basel). 2016 May 12;9(5):360. doi: 10.3390/ma9050360. Materials (Basel). 2016. PMID: 28773485 Free PMC article.
-
New Pyrazolium Salts as a Support for Ionic Liquid Crystals and Ionic Conductors.Materials (Basel). 2018 Apr 3;11(4):548. doi: 10.3390/ma11040548. Materials (Basel). 2018. PMID: 29614030 Free PMC article.
-
T-shaped ionic liquid crystals based on the imidazolium motif: exploring substitution of the C-2 imidazolium carbon atom.Chemistry. 2011 Apr 4;17(15):4291-306. doi: 10.1002/chem.201001921. Epub 2011 Mar 7. Chemistry. 2011. PMID: 21384444
-
Ionic Liquid Crystals as Chromogenic Materials.Materials (Basel). 2024 Sep 17;17(18):4563. doi: 10.3390/ma17184563. Materials (Basel). 2024. PMID: 39336305 Free PMC article. Review.
Cited by
-
Chasing Self-Assembly of Thioether-Substituted Flavylium Salts in Solution and Bulk State.Chemphyschem. 2022 Jul 5;23(13):e202200154. doi: 10.1002/cphc.202200154. Epub 2022 May 17. Chemphyschem. 2022. PMID: 35446455 Free PMC article.
-
Multi-stimuli-responsive polymers enabled by bio-inspired dynamic equilibria of flavylium chemistry.Chem Sci. 2025 Mar 12;16(19):8247-8261. doi: 10.1039/d5sc00977d. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40134655 Free PMC article.
-
Liquid crystal behavior, photoluminescence and gas sensing: A new series of ionic liquid crystal imidazole and benzoimidazole bearing chalcone groups, synthesis and characterization.RSC Adv. 2021 Nov 30;11(61):38444-38456. doi: 10.1039/d1ra07731g. eCollection 2021 Nov 29. RSC Adv. 2021. PMID: 35493216 Free PMC article.
References
-
- Binnemans K., Chem. Rev. 2005, 105, 4148–4204. - PubMed
-
- Goossens K., Lava K., Bielawski C. W., Binnemans K., Chem. Rev. 2016, 116, 4643–4807. - PubMed
-
- Handbook of Liquid Crystals (Eds.: J W. Goodby, C. Tschierske, P. Raynes, H. Gleeson, T. Kato, P. J. Collings), Wiley-VCH, Weinheim, 2014.
-
- Chen S., Eichhorn S. H., Isr. J. Chem. 2012, 52, 830–843.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

