Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro
- PMID: 31419250
- PMCID: PMC6697317
- DOI: 10.1371/journal.pone.0221344
Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro
Abstract
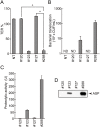
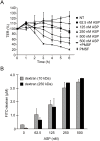
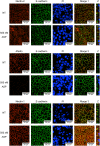
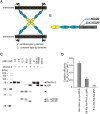
Aeromonas sobria is a pathogen causing food-borne illness. In immunocompromised patients and the elderly, A. sobria can leave the intestinal tract, and this opportunistically leads to severe extraintestinal diseases including sepsis, peritonitis, and meningitis. To cause such extraintestinal diseases, A. sobria must pass through the intestinal epithelial barrier. The mechanism of such bacterial translocation has not been established. Herein we used intestinal (T84) cultured cells to investigate the effect of A. sobria serine protease (ASP) on junctional complexes that maintain the intercellular adhesion of the intestinal epithelium. When several A. sobria strains were inoculated into T84 monolayer grown on Transwell inserts, the strain with higher ASP production largely decreased the value of transepithelial electrical resistance exhibited by the T84 monolayer and markedly caused bacterial translocation from the apical surface into the basolateral side of T84 monolayer. Further experiments revealed that ASP acts on adherens junctions (AJs) and causes the destruction of both nectin-2 and afadin, which are protein components constituting AJs. Other studies have not revealed the bacterial pathogenic factors that cause the destruction of both nectin-2 and afadin, and our present results thus provide the first report that the bacterial extracellular protease ASP affects these molecules. We speculate that the destruction of nectin-2 and afadin by the action of ASP increases the ability of A. sobria to pass through intestinal epithelial tissue and contributes to the severity of pathological conditions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

