Increased mistranslation protects E. coli from protein misfolding stress due to activation of a RpoS-dependent heat shock response
- PMID: 31419308
- PMCID: PMC6878130
- DOI: 10.1002/1873-3468.13578
Increased mistranslation protects E. coli from protein misfolding stress due to activation of a RpoS-dependent heat shock response
Abstract
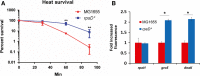
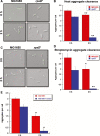
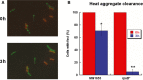
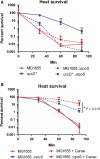
The misincorporation of an incorrect amino acid into a polypeptide during protein synthesis is considered a detrimental phenomenon. A mistranslated protein is often misfolded and degraded or nonfunctional and results in an increased cost to quality control machinery. Despite these costs, errors during protein synthesis are common in bacteria. Here, we report that mistranslation in Escherichia coli increase the protein level of the heat shock sigma factor RpoH and protect cells against heat stress. Surprisingly, this increase in RpoH due to mistranslation is dependent on the presence of the general stress response sigma factor RpoS. This report provides evidence for a protective function of mistranslation and suggests a novel regulatory role of RpoS in the heat shock response.
Keywords: RpoH; RpoS; heat shock response; mistranslation.
© 2019 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures

References
-
- Grossman AD, Erickson JW and Gross CA (1984) The htpR gene product of E. coli is a sigma factor for heat‐shock promoters. Cell 38, 383–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

