Transgenic Expression of PRSS1R122H Sensitizes Mice to Pancreatitis
- PMID: 31419436
- PMCID: PMC7580257
- DOI: 10.1053/j.gastro.2019.08.016
Transgenic Expression of PRSS1R122H Sensitizes Mice to Pancreatitis
Abstract
Background & aims: Mutations in the trypsinogen gene (PRSS1) cause human hereditary pancreatitis. However, it is not clear how mutant forms of PRSS1 contribute to disease development. We studied the effects of expressing mutant forms of human PRSS1 in mice.
Methods: We expressed forms of PRSS1 with and without the mutation encoding R122H (PRSS1R122H) specifically in pancreatic acinar cells under control of a full-length pancreatic elastase gene promoter. Mice that did not express these transgenes were used as controls. Mice were given injections of caerulein to induce acute pancreatitis or injections of lipopolysaccharide to induce chronic pancreatitis. Other groups of mice were fed ethanol or placed on a high-fat diet to induce pancreatitis. Pancreata were collected and analyzed by histology, immunoblots, real-time polymerase chain reaction, and immunohistochemistry. Trypsin enzymatic activity and chymotrypsin enzymatic activity were measured in pancreatic homogenates. Blood was collected and serum amylase activity was measured.
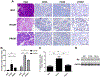
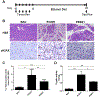
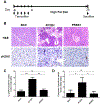
Results: Pancreata from mice expressing transgenes encoding PRSS1 or PRSS1R122H had focal areas of inflammation; these lesions were more prominent in mice that express PRSS1R122H. Pancreata from mice that express PRSS1 or PRSS1R122H had increased levels of heat shock protein 70 and nuclear factor (erythroid-derived 2)-like 2, and reduced levels of chymotrypsin C compared with control mice. Increased expression of PRSS1 or PRSS1R122H increased focal damage in pancreatic tissues and increased the severity of acute pancreatitis after caerulein injection. Administration of lipopolysaccharide exacerbated inflammation in mice that express PRSS1R122H compared to mice that express PRSS1 or control mice. Mice that express PRSS1R122H developed more severe pancreatitis after ethanol feeding or a high-fat diet than mice that express PRSS1 or control mice. Pancreata from mice that express PRSS1R122H had more DNA damage, apoptosis, and collagen deposition and increased trypsin activity and infiltration by inflammatory cells than mice that express PRSS1 or control mice.
Conclusions: Expression of a transgene encoding PRSS1R122H in mice promoted inflammation and increased the severity of pancreatitis compared with mice that express PRSS1 or control mice. These mice might be used as a model for human hereditary pancreatitis and can be studied to determine mechanisms of induction of pancreatitis by lipopolysaccharide, ethanol, or a high-fat diet.
Keywords: Animal Model; Digestive Enzyme; Endotoxin; Immune Cells.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
The Complex Role of Trypsin in Pancreatitis.Gastroenterology. 2020 Mar;158(4):822-826. doi: 10.1053/j.gastro.2019.12.025. Epub 2020 Jan 3. Gastroenterology. 2020. PMID: 31911102 No abstract available.
References
-
- Chiari H Über die Selbstverdauung des menschlichen Pankreas. Zeitschrift für Heilkunde 1896; 17:69–96.
-
- Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996; 14:141–145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

