Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development
- PMID: 31419514
- PMCID: PMC6880766
- DOI: 10.1016/j.jhep.2019.08.005
Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development
Abstract
Background & aims: Fatty liver disease, including non-alcoholic fatty liver (NAFLD) and steatohepatitis (NASH), has been associated with increased intestinal barrier permeability and translocation of bacteria or bacterial products into the blood circulation. In this study, we aimed to unravel the role of both intestinal barrier integrity and microbiota in NAFLD/NASH development.
Methods: C57BL/6J mice were fed with high-fat diet (HFD) or methionine-choline-deficient diet for 1 week or longer to recapitulate aspects of NASH (steatosis, inflammation, insulin resistance). Genetic and pharmacological strategies were then used to modulate intestinal barrier integrity.
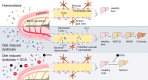
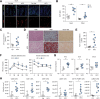
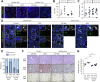
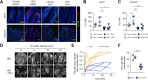
Results: We show that disruption of the intestinal epithelial barrier and gut vascular barrier (GVB) are early events in NASH pathogenesis. Mice fed HFD for only 1 week undergo a diet-induced dysbiosis that drives GVB damage and bacterial translocation into the liver. Fecal microbiota transplantation from HFD-fed mice into specific pathogen-free recipients induces GVB damage and epididymal adipose tissue enlargement. GVB disruption depends on interference with the WNT/β-catenin signaling pathway, as shown by genetic intervention driving β-catenin activation only in endothelial cells, preventing GVB disruption and NASH development. The bile acid analogue and farnesoid X receptor agonist obeticholic acid (OCA) drives β-catenin activation in endothelial cells. Accordingly, pharmacologic intervention with OCA protects against GVB disruption, both as a preventive and therapeutic agent. Importantly, we found upregulation of the GVB leakage marker in the colon of patients with NASH.
Conclusions: We have identified a new player in NASH development, the GVB, whose damage leads to bacteria or bacterial product translocation into the blood circulation. Treatment aimed at restoring β-catenin activation in endothelial cells, such as administration of OCA, protects against GVB damage and NASH development.
Lay summary: The incidence of fatty liver disease is reaching epidemic levels in the USA, with more than 30% of adults having NAFLD (non-alcoholic fatty liver disease), which can progress to more severe non-alcoholic steatohepatitis (NASH). Herein, we show that disruption of the intestinal epithelial barrier and gut vascular barrier are early events in the development of NASH. We show that the drug obeticholic acid protects against barrier disruption and thereby prevents the development of NASH, providing further evidence for its use in the prevention or treatment of NASH.
Copyright © 2019 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

