Bleomycin Induces Drug Efflux in Lungs. A Pitfall for Pharmacological Studies of Pulmonary Fibrosis
- PMID: 31419911
- PMCID: PMC6993545
- DOI: 10.1165/rcmb.2018-0147OC
Bleomycin Induces Drug Efflux in Lungs. A Pitfall for Pharmacological Studies of Pulmonary Fibrosis
Abstract
ATP-binding cassette (ABC) transporters are evolutionarily conserved membrane proteins that pump a variety of endogenous substrates across cell membranes. Certain subfamilies are known to interact with pharmaceutical compounds, potentially influencing drug delivery and treatment efficacy. However, the role of drug resistance-associated ABC transporters has not been examined in idiopathic pulmonary fibrosis (IPF) or its animal model: the bleomycin (BLM)-induced murine model. Here, we investigate the expression of two ABC transporters, P-gp (permeability glycoprotein) and BCRP (breast cancer resistance protein), in human IPF lung tissue and two different BLM-induced mouse models of pulmonary fibrosis. We obtained human IPF specimens from patients during lung transplantation and administered BLM to male C57BL/6J mice either by oropharyngeal aspiration (1 U/kg) or subcutaneous osmotic infusion (100 U/kg over 7 d). We report that P-gp and BCRP expression in lungs of patients with IPF was comparable to controls. However, murine lungs expressed increased levels of P-gp and BCRP after oropharyngeal and subcutaneous BLM administration. We localized this upregulation to multiple pulmonary cell types, including alveolar fibroblasts, endothelial cells, and type 2 epithelial cells. Functionally, this effect reduced murine lung exposure to nintedanib, a U.S. Food and Drug Administration-approved IPF therapy known to be a P-gp substrate. The study reveals a discrepancy between IPF pathophysiology and the common animal model of lung fibrosis. BLM-induced drug efflux in the murine lungs may present an uncontrolled confounding variable in the preclinical study of IPF drug candidates, and these findings will facilitate disease model validation and enhance new drug discoveries that will ultimately improve patient outcomes.
Keywords: ATP-binding cassette transporters; animal models; bleomycin; drug resistance; idiopathic pulmonary fibrosis.
Figures


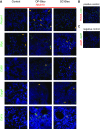

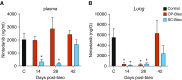


Comment in
-
ABC Transporters: An Overlooked Mechanism of Drug Failure in Our Preclinical Models?Am J Respir Cell Mol Biol. 2020 Feb;62(2):130-131. doi: 10.1165/rcmb.2019-0284ED. Am J Respir Cell Mol Biol. 2020. PMID: 31469577 Free PMC article. No abstract available.
Similar articles
-
Peripheral Hybrid CB1R and iNOS Antagonist MRI-1867 Displays Anti-Fibrotic Efficacy in Bleomycin-Induced Skin Fibrosis.Front Endocrinol (Lausanne). 2021 Sep 28;12:744857. doi: 10.3389/fendo.2021.744857. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34650521 Free PMC article.
-
Nintedanib Ameliorates Bleomycin-Induced Pulmonary Fibrosis, Inflammation, Apoptosis, and Oxidative Stress by Modulating PI3K/Akt/mTOR Pathway in Mice.Inflammation. 2023 Aug;46(4):1531-1542. doi: 10.1007/s10753-023-01825-2. Epub 2023 May 9. Inflammation. 2023. PMID: 37160579 Free PMC article.
-
Nintedanib Reduces Neutrophil Chemotaxis via Activating GRK2 in Bleomycin-Induced Pulmonary Fibrosis.Int J Mol Sci. 2020 Jul 2;21(13):4735. doi: 10.3390/ijms21134735. Int J Mol Sci. 2020. PMID: 32630825 Free PMC article.
-
The Chemokine System as a Key Regulator of Pulmonary Fibrosis: Converging Pathways in Human Idiopathic Pulmonary Fibrosis (IPF) and the Bleomycin-Induced Lung Fibrosis Model in Mice.Cells. 2024 Dec 12;13(24):2058. doi: 10.3390/cells13242058. Cells. 2024. PMID: 39768150 Free PMC article. Review.
-
The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis.Exp Lung Res. 2015 Mar;41(2):57-73. doi: 10.3109/01902148.2014.979516. Epub 2014 Dec 16. Exp Lung Res. 2015. PMID: 25514507 Review.
Cited by
-
Extracellular CIRP Induces an Inflammatory Phenotype in Pulmonary Fibroblasts via TLR4.Front Immunol. 2021 Jul 23;12:721970. doi: 10.3389/fimmu.2021.721970. eCollection 2021. Front Immunol. 2021. PMID: 34367191 Free PMC article.
-
Chronic and Binge Alcohol Ingestion Increases Truncated Oxidized Phosphatidylcholines in Mice Lungs Due to Increased Oxidative Stress.Front Physiol. 2022 May 24;13:860449. doi: 10.3389/fphys.2022.860449. eCollection 2022. Front Physiol. 2022. PMID: 35685280 Free PMC article.
-
CB1 R and iNOS are distinct players promoting pulmonary fibrosis in Hermansky-Pudlak syndrome.Clin Transl Med. 2021 Jul;11(7):e471. doi: 10.1002/ctm2.471. Clin Transl Med. 2021. PMID: 34323400 Free PMC article.
-
Update in Interstitial Lung Disease 2020.Am J Respir Crit Care Med. 2021 Jun 1;203(11):1343-1352. doi: 10.1164/rccm.202103-0559UP. Am J Respir Crit Care Med. 2021. PMID: 33835899 Free PMC article. Review. No abstract available.
-
Animal models of drug-induced pulmonary fibrosis: an overview of molecular mechanisms and characteristics.Cell Biol Toxicol. 2022 Oct;38(5):699-723. doi: 10.1007/s10565-021-09676-z. Epub 2021 Nov 5. Cell Biol Toxicol. 2022. PMID: 34741237 Review.
References
-
- Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;379:797–798. - PubMed
-
- Jenkins RG, Moore BB, Chambers RC, Eickelberg O, Königshoff M, Kolb M, et al. ATS Assembly on Respiratory Cell and Molecular Biology. An Official American Thoracic Society Workshop Report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;56:667–679. - PMC - PubMed
-
- Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017;1627:27–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

