miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt-β-catenin pathway
- PMID: 31419987
- PMCID: PMC6697940
- DOI: 10.1186/s13046-019-1370-1
miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt-β-catenin pathway
Abstract
Background: MicroRNAs (miRNAs) play crucial roles in tumor initiation and development. Previously, we indicated that miR-504 is downregulated and suppresses tumor proliferation in glioblastoma (GBM). However, the regulation and relevant mechanism of miR-504 in GBM mesenchymal (ME) transition remain unclear.
Methods: Transcriptome and clinical data were obtained from The Cancer Genome Atlas (TCGA) database. The potential functions of miR-504 were predicted using gene ontology analysis. GBM cell migration and invasion were examined using wound healing and Transwell assays. Epithelial-mesenchymal transition (EMT) progression in GBM cell lines was detected with immunofluorescence and western blotting. The stemness activity of glioma stem-like cells (GSCs) was assessed by sphere formation assay and tumor xenograft model. miR-504 binding to the FZD7 (frizzled class receptor 7) 3' untranslated region (3'UTR) was validated using dual luciferase reporter assay. TOP/FOP Flash assays were conducted to determine the effects of miR-504 on Wnt/β-catenin signaling.
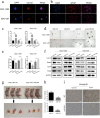
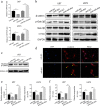
Results: Analysis of TCGA transcriptomic data showed that low miR-504 expression correlated with ME subtype transition and poor survival in patients with GBM. Functional experiments showed that miR-504 overexpression suppressed malignant behaviors of GBM cells, such as migration, invasion, EMT, and stemness activity. Furthermore, miR-504 was a negative regulator of the Wnt-β-catenin pathway by directly repressing FZD7 expression, and FZD7 overexpression reversed the EMT inhibition caused by miR-504. Moreover, the low miR-504/FZD7 expression ratio was a ME subtype marker and could serve as a significant prognostic indicator and predict the clinical outcome of chemotherapy and radiotherapy for patients with GBM in TCGA dataset.
Conclusions: Our results suggest that miR-504 suppresses the aggressive biological processes associated with the ME phenotype of GBM and could be a potential candidate for therapeutic applications in these malignant brain tumors.
Keywords: EMT; FZD7; GBM; Mesenchymal phenotype; Wnt–β-catenin pathway; miR-504.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

