A pathogenic role of plasmacytoid dendritic cells in autoimmunity and chronic viral infection
- PMID: 31420375
- PMCID: PMC6719431
- DOI: 10.1084/jem.20181359
A pathogenic role of plasmacytoid dendritic cells in autoimmunity and chronic viral infection
Abstract
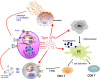
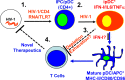
Following the discovery of plasmacytoid dendritic cells (pDCs) and of their extraordinary ability to produce type I IFNs (IFN-I) in response to TLR7 and TLR9 stimulation, it is assumed that their main function is to participate in the antiviral response. There is increasing evidence suggesting that pDCs and/or IFN-I can also have a detrimental role in a number of inflammatory and autoimmune diseases, in the context of chronic viral infections and in cancers. Whether these cells should be targeted in patients and how much of their biology is connected to IFN-I production remains unclear and is discussed here.
Figures

References
-
- Alcántara-Hernández M., Leylek R., Wagar L.E., Engleman E.G., Keler T., Marinkovich M.P., Davis M.M., Nolan G.P., and Idoyaga J.. 2017. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 47:1037–1050.e6. 10.1016/j.immuni.2017.11.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

