Efficacy and safety of the combination of reduced duration prophylaxis followed by immuno-guided prophylaxis to prevent cytomegalovirus disease in lung transplant recipients (CYTOCOR STUDY): an open-label, randomised, non-inferiority clinical trial
- PMID: 31420397
- PMCID: PMC6701703
- DOI: 10.1136/bmjopen-2019-030648
Efficacy and safety of the combination of reduced duration prophylaxis followed by immuno-guided prophylaxis to prevent cytomegalovirus disease in lung transplant recipients (CYTOCOR STUDY): an open-label, randomised, non-inferiority clinical trial
Abstract
Introduction: Prolonged use of antivirals to prevent the development of cytomegalovirus (CMV) disease in lung transplant patients has been shown to have significant side effects, for which alternatives are being sought to reduce their use. The monitoring of cell immunity against CMV could be an alternative as it has shown to be useful in identifying transplant patients at low risk of infection, who could benefit from shorter prophylaxis. The aim of the CYTOCOR study is to demonstrate that the combination of a reduced prophylaxis strategy with subsequent CMV-specific immunological monitoring would allow CMV infection to be controlled in lung transplant patients as effectively as the usual strategy (prophylaxis followed by pre-emptive therapy), while reducing the side effects of antivirals due to the shorter duration of prophylaxis.
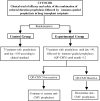
Methods and analysis: Phase III randomised, open, multicentre, parallel, non-inferiority clinical trial to study the efficacy and safety of the combination of a prophylaxis strategy up to month +3 post-transplant followed by immuno-guided prophylaxis using the QuantiFERON-CMV technique up to month +12 post-transplant to prevent CMV disease in CMV-seropositive lung transplant recipients. This strategy will be compared with a combination of a usual prophylaxis strategy up to month +6 post-transplant followed by pre-emptive therapy up to month +12. To study the incidence of CMV disease, patients will be followed up to 18 months post-transplantation. A total of 150 patients are expected to be recruited for the study.
Ethics and public dissemination: The clinical trial has been approved by the Research Ethics Committees and authorised by the Spanish Agency of Medicines and Medical Devices (AEMPS).If the hypothesis of this clinical trial is verified, the dissemination of the results could change clinical practice by increasing knowledge about the safety and efficacy of discontinuing valganciclovir prophylaxis in lung transplant recipients.
Trial registration number: NCT03699254.
Keywords: cytomegalovirus; immuno-guided prophylaxis; lung transplantation.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: J.T.C. and S.C. have received an unrestricted research grant from Roche Pharma and Qiagen. They have also received payments from Qiagen for educational activities.
Figures
References
-
- Kotton CN, Kumar D, Caliendo AM, et al. . The transplantation Society international CMV consensus group. The third International consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018;102:900–31. - PubMed
-
- Manuel O, Husain S, Kumar D, et al. . Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clinical Infectious Diseases 2013;56:817–24. 10.1093/cid/cis993 - DOI - PubMed