Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells
- PMID: 31420514
- PMCID: PMC6731669
- DOI: 10.1073/pnas.1906512116
Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells
Abstract
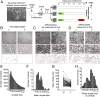
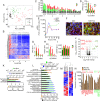
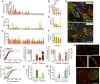
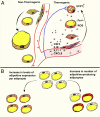
Single-cell sequencing technologies have revealed an unexpectedly broad repertoire of cells required to mediate complex functions in multicellular organisms. Despite the multiple roles of adipose tissue in maintaining systemic metabolic homeostasis, adipocytes are thought to be largely homogenous with only 2 major subtypes recognized in humans so far. Here we report the existence and characteristics of 4 distinct human adipocyte subtypes, and of their respective mesenchymal progenitors. The phenotypes of these distinct adipocyte subtypes are differentially associated with key adipose tissue functions, including thermogenesis, lipid storage, and adipokine secretion. The transcriptomic signature of "brite/beige" thermogenic adipocytes reveals mechanisms for iron accumulation and protection from oxidative stress, necessary for mitochondrial biogenesis and respiration upon activation. Importantly, this signature is enriched in human supraclavicular adipose tissue, confirming that these cells comprise thermogenic depots in vivo, and explain previous findings of a rate-limiting role of iron in adipose tissue browning. The mesenchymal progenitors that give rise to beige/brite adipocytes express a unique set of cytokines and transcriptional regulators involved in immune cell modulation of adipose tissue browning. Unexpectedly, we also find adipocyte subtypes specialized for high-level expression of the adipokines adiponectin or leptin, associated with distinct transcription factors previously implicated in adipocyte differentiation. The finding of a broad adipocyte repertoire derived from a distinct set of mesenchymal progenitors, and of the transcriptional regulators that can control their development, provides a framework for understanding human adipose tissue function and role in metabolic disease.
Keywords: adipocyte differentiation; brown adipocyte; human adipose tissue; mesenchymal stem cells; progenitor cells.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kusminski C. M., Bickel P. E., Scherer P. E., Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 15, 639–660 (2016). - PubMed
-
- Nedergaard J., Cannon B., Brown adipose tissue as a heat-producing thermoeffector. Handb. Clin. Neurol. 156, 137–152 (2018). - PubMed
-
- Cinti S., The adipose organ: Morphological perspectives of adipose tissues. Proc. Nutr. Soc. 60, 319–328 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

