Generation of amine dehydrogenases with increased catalytic performance and substrate scope from ε-deaminating L-Lysine dehydrogenase
- PMID: 31420547
- PMCID: PMC6697735
- DOI: 10.1038/s41467-019-11509-x
Generation of amine dehydrogenases with increased catalytic performance and substrate scope from ε-deaminating L-Lysine dehydrogenase
Abstract
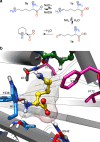
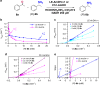
Amine dehydrogenases (AmDHs) catalyse the conversion of ketones into enantiomerically pure amines at the sole expense of ammonia and hydride source. Guided by structural information from computational models, we create AmDHs that can convert pharmaceutically relevant aromatic ketones with conversions up to quantitative and perfect chemical and optical purities. These AmDHs are created from an unconventional enzyme scaffold that apparently does not operate any asymmetric transformation in its natural reaction. Additionally, the best variant (LE-AmDH-v1) displays a unique substrate-dependent switch of enantioselectivity, affording S- or R-configured amine products with up to >99.9% enantiomeric excess. These findings are explained by in silico studies. LE-AmDH-v1 is highly thermostable (Tm of 69 °C), retains almost entirely its catalytic activity upon incubation up to 50 °C for several days, and operates preferentially at 50 °C and pH 9.0. This study also demonstrates that product inhibition can be a critical factor in AmDH-catalysed reductive amination.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Constable DJC, et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green. Chem. 2007;9:411–420. doi: 10.1039/B703488C. - DOI
-
- Ghislieri D, Turner NJ. Biocatalytic approaches to the synthesis of enantiomerically pure chiral amines. Top. Catal. 2013;57:284–300. doi: 10.1007/s11244-013-0184-1. - DOI
-
- Chiral Amine Synthesis: Methods, Developments and Applications (ed. Nugent, T. C.) (Wiley-VCH, Weinheim, 2010).
-
- Wang, C. & Xiao, J. Asymmetric reductive amination, in Stereoselective Formation of Amines Vol. 343 (eds. Wei, L. & Xumu, Z.) 261–282 (Springer-Verlag, Berlin, 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

