Indoor ozone/human chemistry and ventilation strategies
- PMID: 31420890
- PMCID: PMC6856811
- DOI: 10.1111/ina.12594
Indoor ozone/human chemistry and ventilation strategies
Abstract
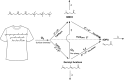
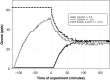
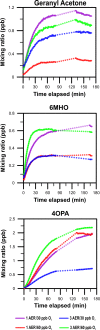
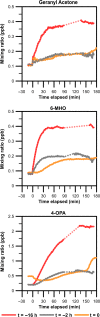
This study aimed to better understand and quantify the influence of ventilation strategies on occupant-related indoor air chemistry. The oxidation of human skin oil constituents was studied in a continuously ventilated climate chamber at two air exchange rates (1 h-1 and 3 h-1 ) and two initial ozone mixing ratios (30 and 60 ppb). Additional measurements were performed to investigate the effect of intermittent ventilation ("off" followed by "on"). Soiled t-shirts were used to simulate the presence of occupants. A time-of-flight-chemical ionization mass spectrometer (ToF-CIMS) in positive mode using protonated water clusters was used to measure the oxygenated reaction products geranyl acetone, 6-methyl-5-hepten-2-one (6-MHO) and 4-oxopentanal (4-OPA). The measurement data were used in a series of mass balance models accounting for formation and removal processes. Reactions of ozone with squalene occurring on the surface of the t-shirts are mass transport limited; ventilation rate has only a small effect on this surface chemistry. Ozone-squalene reactions on the t-shirts produced gas-phase geranyl acetone, which was subsequently removed almost equally by ventilation and further reaction with ozone. About 70% of gas-phase 6-MHO was produced in surface reactions on the t-shirts, the remainder in secondary gas-phase reactions of ozone with geranyl acetone. 6-MHO was primarily removed by ventilation, while further reaction with ozone was responsible for about a third of its removal. 4-OPA was formed primarily on the surfaces of the shirts (~60%); gas-phase reactions of ozone with geranyl acetone and 6-MHO accounted for ~30% and ~10%, respectively. 4-OPA was removed entirely by ventilation. The results from the intermittent ventilation scenarios showed delayed formation of the reaction products and lower product concentrations compared to continuous ventilation.
Keywords: ToF-CIMS; air exchange rate; indoor environment; oxygenated volatile organic compounds; ozone; squalene.
© 2019 The Authors. Indoor Air published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Ozone-initiated chemistry in an occupied simulated aircraft cabin.Environ Sci Technol. 2007 Sep 1;41(17):6177-84. doi: 10.1021/es0708520. Environ Sci Technol. 2007. PMID: 17937299
-
Products of ozone-initiated chemistry in a simulated aircraft environment.Environ Sci Technol. 2005 Jul 1;39(13):4823-32. doi: 10.1021/es047992j. Environ Sci Technol. 2005. PMID: 16053080
-
Heterogeneous oxidation of squalene film by ozone under various indoor conditions.Indoor Air. 2009 Oct;19(5):381-91. doi: 10.1111/j.1600-0668.2009.00599.x. Epub 2009 Feb 13. Indoor Air. 2009. PMID: 19500173
-
The Indoor Chemical Human Emissions and Reactivity (ICHEAR) project: Overview of experimental methodology and preliminary results.Indoor Air. 2020 Nov;30(6):1213-1228. doi: 10.1111/ina.12687. Epub 2020 Jun 7. Indoor Air. 2020. PMID: 32424858 Review.
-
Indoor ozone: Concentrations and influencing factors.Indoor Air. 2022 Jan;32(1):e12942. doi: 10.1111/ina.12942. Epub 2021 Oct 5. Indoor Air. 2022. PMID: 34609012 Review.
Cited by
-
Total OH Reactivity of Emissions from Humans: In Situ Measurement and Budget Analysis.Environ Sci Technol. 2021 Jan 5;55(1):149-159. doi: 10.1021/acs.est.0c04206. Epub 2020 Dec 9. Environ Sci Technol. 2021. PMID: 33295177 Free PMC article.
-
The influence of personal care products on ozone-skin surface chemistry.PLoS One. 2022 Sep 29;17(9):e0268263. doi: 10.1371/journal.pone.0268263. eCollection 2022. PLoS One. 2022. PMID: 36174009 Free PMC article.
-
Emission Rates of Volatile Organic Compounds from Humans.Environ Sci Technol. 2022 Apr 19;56(8):4838-4848. doi: 10.1021/acs.est.1c08764. Epub 2022 Apr 7. Environ Sci Technol. 2022. PMID: 35389619 Free PMC article.
-
Effect of Ozone, Clothing, Temperature, and Humidity on the Total OH Reactivity Emitted from Humans.Environ Sci Technol. 2021 Oct 19;55(20):13614-13624. doi: 10.1021/acs.est.1c01831. Epub 2021 Sep 30. Environ Sci Technol. 2021. PMID: 34591444 Free PMC article.
-
Influence of Ventilation on Formation and Growth of 1-20 nm Particles via Ozone-Human Chemistry.Environ Sci Technol. 2024 Mar 12;58(10):4704-4715. doi: 10.1021/acs.est.3c08466. Epub 2024 Feb 7. Environ Sci Technol. 2024. PMID: 38326946 Free PMC article.
References
-
- Weschler CJ, Shields HC. Potential reactions among indoor pollutants. Atmos Environ. 1997;31:3487‐3495.
-
- Weschler CJ. Chemistry in indoor environments: 20 years of research. Indoor Air. 2011;21:205‐218. - PubMed
-
- Weschler CJ, Carslaw N. Indoor chemistry. Environ Sci Technol. 2018;52:2419‐2428. - PubMed
-
- Tang X, Misztal P, Nazaroff WW, Goldstein AH. Volatile organic compound emissions from humans indoors. Environ Sci Technol. 2016;50:12686‐12694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

