Neurobiology of resilience in depression: immune and vascular insights from human and animal studies
- PMID: 31421056
- PMCID: PMC7891571
- DOI: 10.1111/ejn.14547
Neurobiology of resilience in depression: immune and vascular insights from human and animal studies
Abstract
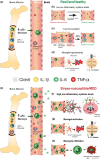
Major depressive disorder (MDD) is a chronic and recurrent psychiatric condition characterized by depressed mood, social isolation and anhedonia. It will affect 20% of individuals with considerable economic impacts. Unfortunately, 30-50% of depressed individuals are resistant to current antidepressant treatments. MDD is twice as prevalent in women and associated symptoms are different. Depression's main environmental risk factor is chronic stress, and women report higher levels of stress in daily life. However, not every stressed individual becomes depressed, highlighting the need to identify biological determinants of stress vulnerability but also resilience. Based on a reverse translational approach, rodent models of depression were developed to study the mechanisms underlying susceptibility vs resilience. Indeed, a subpopulation of animals can display coping mechanisms and a set of biological alterations leading to stress resilience. The aetiology of MDD is multifactorial and involves several physiological systems. Exacerbation of endocrine and immune responses from both innate and adaptive systems are observed in depressed individuals and mice exhibiting depression-like behaviours. Increasing attention has been given to neurovascular health since higher prevalence of cardiovascular diseases is found in MDD patients and inflammatory conditions are associated with depression, treatment resistance and relapse. Here, we provide an overview of endocrine, immune and vascular changes associated with stress vulnerability vs. resilience in rodents and when available, in humans. Lack of treatment efficacy suggests that neuron-centric treatments do not address important causal biological factors and better understanding of stress-induced adaptations, including sex differences, could contribute to develop novel therapeutic strategies including personalized medicine approaches.
Keywords: blood-brain barrier; cytokines; depression; immune; sex differences; stress; vascular.
© 2019 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
-
- Abbott, N. J. , Patabendige, A. A. K. , Dolman, D. E. M. , Yusof, S. R. , & Begley, D. J. (2010). Structure and function of the blood‐brain barrier. Neurobiology of Diseases, 37, 13–25. - PubMed
-
- Abdullah, M. , Chai, P.‐S. , Chong, M.‐Y. , Tohit, E. R. M. , Ramasamy, R. , Pei, C. P. , & Vidyadaran, S. (2012). Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cellular Immunology, 272, 214–219. - PubMed
-
- Ajami, B. , Bennett, J. L. , Krieger, C. , Tetzlaff, W. , & Rossi, F. M. V. (2007). Local self‐renewal can sustain CNS microglia maintenance and function throughout adult life. Nature Neuroscience, 10, 1538–1543. - PubMed
-
- Ajami, B. , Bennett, J. L. , Krieger, C. , McNagny, K. M. , & Rossi, F. M. V. (2011). Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neuroscience, 14, 1142–1149. - PubMed
-
- Altshuler, L. L. , Abulseoud, O. A. , Foland‐Ross, L. , Bartzokis, G. , Chang, S. , Mintz, J. , … Vinters, H. V. (2010). Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disorder, 12, 541–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

