Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis
- PMID: 31422028
- PMCID: PMC7193685
- DOI: 10.1016/S1470-2045(19)30407-3
Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis
Abstract
Background: Phase 3 clinical data has shown higher proportions of patients with objective response, longer response duration, and longer overall survival with nivolumab versus docetaxel in patients with previously treated advanced non-small-cell lung cancer (NSCLC). We aimed to evaluate the long-term benefit of nivolumab and the effect of response and disease control on subsequent survival.
Methods: We pooled data from four clinical studies of nivolumab in patients with previously treated NSCLC (CheckMate 017, 057, 063, and 003) to evaluate survival outcomes. Trials of nivolumab in the second-line or later setting with at least 4 years follow-up were included. Comparisons of nivolumab versus docetaxel included all randomised patients from the phase 3 CheckMate 017 and 057 studies. We did landmark analyses by response status at 6 months to determine post-landmark survival outcomes. We excluded patients who did not have a radiographic tumour assessment at 6 months. Safety analyses included all patients who received at least one dose of nivolumab.
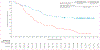
Findings: Across all four studies, 4-year overall survival with nivolumab was 14% (95% CI 11-17) for all patients (n=664), 19% (15-24) for those with at least 1% PD-L1 expression, and 11% (7-16) for those with less than 1% PD-L1 expression. In CheckMate 017 and 057, 4-year overall survival was 14% (95% CI 11-18) in patients treated with nivolumab, compared with 5% (3-7) in patients treated with docetaxel. Survival subsequent to response at 6 months on nivolumab or docetaxel was longer than after progressive disease at 6 months, with hazard ratios for overall survival of 0·18 (95% 0·12-0·27) for nivolumab and 0·43 (0·29-0·65) for docetaxel; for stable disease versus progressive disease, hazard ratios were 0·52 (0·37-0·71) for nivolumab and 0·80 (0·61-1·04) for docetaxel. Long-term data did not show any new safety signals.
Interpretation: Patients with advanced NSCLC treated with nivolumab achieved a greater duration of response compared with patients treated with docetaxel, which was associated with a long-term survival advantage.
Funding: Bristol-Myers Squibb.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
SJA is on the advisory board or scientific advisory board of Bristol-Myers Squibb, Novartis, Merck, CBMG, Boehringer Ingelheim, AstraZeneca/MedImmune, Memgen, FLX Bio, Nektar, and Veen; has done contracted research for Novartis; was previously employed by H Lee Moffitt Cancer Center & Research Institute (Tampa, FL, USA); and is currently employed by Duke Cancer Institute (Durham, NC, USA). HB reports grants from Bristol-Myers Squibb, Celgene, Lilly, Merck, and Millennium; personal fees for consultancy or advisory board services (or both) from Bristol-Myers Squibb, Celgene, Genentech, Lilly, EMD-Serono, Merck, Millennium, Pfizer, Boehringer Ingelheim, AstraZeneca, Genmab, Novartis, Regeneron, BioNTech, Cantargia AB, Amgen, and Axiom; has provided educational services for AbbVie and Axiom; and has served on a Data and Safety Monitoring Board for Takeda. SSR reports grants from AstraZeneca, Merck and Tesaro, and personal fees for consultant or advisory board services from Amgen, AbbVie, Bristol-Myers Squibb, Lilly, Genentech, Takeda, and Luxo. LH reports grants from Boehringer Ingelheim and Xcovery; non-financial support from Xcovery; and personal fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, Merck, Genentech, Incyte, EMD Serono, and Tesaro. JDCC reports personal fees from Bristol-Myers Squibb, AstraZeneca, MSD, and Roche. AP reports personal fees from Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, MSD, Pfizer, and Roche, and non-financial support from Bristol-Myers Squibb, Boehringer Ingelheim, and Roche. MG reports personal fees from Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Otsuka Pharma, Celgene, Roche, Pfizer, Incyte, Inivata, and Takeda, and has served as principal investigator or conducted patient enrolment in clinical trials (or both) for Bristol-Myers Squibb, AstraZeneca, Eli Lilly, MSD, Novartis, Otsuka Pharma, Celgene, Roche, Pfizer, Tiziana Sciences, Clovis, Merck Serono, and Bayer. LQMC reports grants from Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, Novartis, Merck, Lilly/ImClone, Incyte, Pfizer, VentiRx, Seattle Genetics and Dynavax; and personal fees from Bristol-Myers Squibb, Amgen, AstraZeneca/MedImmune, Genentech, Novartis, Merck, Pfizer, Sanofi-Genzyme, Seattle Genetics, Synthorx, Takeda, and Dynavax. SG served as a consultant or advisor (or both) to Bristol-Myers Squibb and NEKTAR; reports research funding from Bristol-Myers Squibb; and reports financial support to his institution from Iovance, Takeda/ARIAD and Genentech for clinical trials. DP reports honoraria from Bristol-Myers Squibb, AstraZeneca, Boehringer, Celgene, Novartis, Pfizer, MSD, Roche, prIME Oncology, and peer CME for services as a consultant, advisor, or lecturer; travel expenses from AstraZeneca, Roche, Novartis, prIME Oncology, and Pfizer, and financial support to his institution from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmune, Sanofi-Aventis, Taiho Pharma, Novocure, and Daiichi Sankyo for clinical trials. CB reports honoraria from Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Merck, and Pfizer. AD reports honoraria from Medscape, Onclive, PeerVoice, Physicians Education Resources, Tyra Biosciences, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview, AstraZeneca, Genentech and Bayer, and has served as a consultant or advisor to Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, BluePrint Medicines, Genentech, Takeda, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, and Bayer. GAO reports grants from Bristol-Myers Squibb, AstraZeneca, Merck, Genentech, and Pfizer, and consultant fees for his institution from Merck, Novartis, Pfizer and Takeda. SA and JRP are employed by and own stocks in Bristol-Myers Squibb. AL is employed by Bristol-Myers Squibb. JB reports grants and travel expenses from Bristol-Myers Squibb and personal fees from AstraZeneca, Genentech, and Merck. MAB, LC, and JW-T declare no competing interests.
Figures




Comment in
-
Immune checkpoint inhibitors: a game changer for metastatic non-small-cell lung cancer.Lancet Oncol. 2019 Oct;20(10):1334-1335. doi: 10.1016/S1470-2045(19)30508-X. Epub 2019 Aug 14. Lancet Oncol. 2019. PMID: 31422027 No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. - PubMed
-
- Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. https://seer.cancer.gov/csr/1975_2015/ (accessed Feb 15, 2019).
-
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

