All-cause mortality versus cancer-specific mortality as outcome in cancer screening trials: A review and modeling study
- PMID: 31422585
- PMCID: PMC6792501
- DOI: 10.1002/cam4.2476
All-cause mortality versus cancer-specific mortality as outcome in cancer screening trials: A review and modeling study
Abstract
Background: All-cause mortality has been suggested as an end-point in cancer screening trials in order to avoid biases in attributing the cause of death. The aim of this study was to investigate which sample size and follow-up is needed to find a significant reduction in all-cause mortality.
Methods: A literature review was conducted to identify previous studies that modeled the effect of screening on all-cause mortality. Microsimulation modeling was used to simulate breast cancer, lung cancer, and colorectal cancer screening trials. Model outputs were: cancer-specific deaths, all-cause deaths, and life-years gained per year of follow-up.
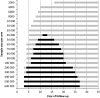
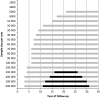
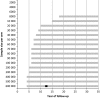
Results: There were large differences between the evaluated cancers. For lung cancer, when 40 000 high-risk people are randomized to each arm, a significant reduction in all-cause mortality could be expected between 11 and 13 years of follow-up. For breast cancer, a significant reduction could be found between 16 and 26 years of follow-up for a sample size of over 300 000 women in each arm. For colorectal cancer, 600 000 persons in each arm were required to be followed for 15-20 years. Our systematic literature review identified seven papers, which showed highly similar results to our estimates.
Conclusion: Cancer screening trials are able to demonstrate a significant reduction in all-cause mortality due to screening, but require very large sample sizes. Depending on the cancer, 40 000-600 000 participants per arm are needed to demonstrate a significant reduction. The reduction in all-cause mortality can only be detected between specific years of follow-up, more limited than the timeframe to detect a reduction in cancer-specific mortality.
Keywords: breast; cancer screening; colorectal; evaluation; lung; mortality reduction; trial.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare: Dr Heijnsdijk, Dr Gini, Csanádi, Bendes, Dr Senore, Dr Anttila report grants from EU‐Framework Programme (Horizon 2020; Number 634753, PI: H.J. de Koning) of the European Commission, during the conduct of the study. Dr ten Haaf and Dr de Koning report grants and nonfinancial support from NELSON‐Netherlands‐Leuven Lung Cancer Screening, grants from NIH/National Cancer Institute, nonfinancial support from International Association for the Study of Lung Cancer Strategic Screening Advisory Committee, grants from Sunnybrook Health Sciences, Toronto, Canada, grants from University of Zurich, Switzerland, outside the submitted work.
Figures
References
-
- Black WC, Haggstrom DA, Welch HG. All‐cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94(3):167‐173. - PubMed
-
- Interventions IWGotEoC‐P , ed. Breast cancer screening. Lyon: International Agency for Research on Cancer; 2016. - PubMed
-
- Penston J. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? Yes. BMJ. 2011;343:d6395. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

