Rare De Novo Missense Variants in RNA Helicase DDX6 Cause Intellectual Disability and Dysmorphic Features and Lead to P-Body Defects and RNA Dysregulation
- PMID: 31422817
- PMCID: PMC6731366
- DOI: 10.1016/j.ajhg.2019.07.010
Rare De Novo Missense Variants in RNA Helicase DDX6 Cause Intellectual Disability and Dysmorphic Features and Lead to P-Body Defects and RNA Dysregulation
Abstract
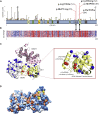
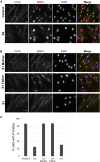
The human RNA helicase DDX6 is an essential component of membrane-less organelles called processing bodies (PBs). PBs are involved in mRNA metabolic processes including translational repression via coordinated storage of mRNAs. Previous studies in human cell lines have implicated altered DDX6 in molecular and cellular dysfunction, but clinical consequences and pathogenesis in humans have yet to be described. Here, we report the identification of five rare de novo missense variants in DDX6 in probands presenting with intellectual disability, developmental delay, and similar dysmorphic features including telecanthus, epicanthus, arched eyebrows, and low-set ears. All five missense variants (p.His372Arg, p.Arg373Gln, p.Cys390Arg, p.Thr391Ile, and p.Thr391Pro) are located in two conserved motifs of the RecA-2 domain of DDX6 involved in RNA binding, helicase activity, and protein-partner binding. We use functional studies to demonstrate that the first variants identified (p.Arg373Gln and p.Cys390Arg) cause significant defects in PB assembly in primary fibroblast and model human cell lines. These variants' interactions with several protein partners were also disrupted in immunoprecipitation assays. Further investigation via complementation assays included the additional variants p.Thr391Ile and p.Thr391Pro, both of which, similarly to p.Arg373Gln and p.Cys390Arg, demonstrated significant defects in P-body assembly. Complementing these molecular findings, modeling of the variants on solved protein structures showed distinct spatial clustering near known protein binding regions. Collectively, our clinical and molecular data describe a neurodevelopmental syndrome associated with pathogenic missense variants in DDX6. Additionally, we suggest DDX6 join the DExD/H-box genes DDX3X and DHX30 in an emerging class of neurodevelopmental disorders involving RNA helicases.
Keywords: DDX6; DEAD-box; DExD/H-box; RNA helicase; RecA domain; helicase; intellectual disability; mRNA metabolism; missense variants; neurodevelopmental disorder; p-bodies; processing bodies.
Copyright © 2019 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
I.W., K.G.M., and K.M. are employees of GeneDx, Inc.
Figures



References
-
- Vissers L.E.L.M., Gilissen C., Veltman J.A. Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 2016;17:9–18. - PubMed
-
- Bardoni B., Abekhoukh S., Zongaro S., Melko M. Intellectual disabilities, neuronal posttranscriptional RNA metabolism, and RNA-binding proteins: Three actors for a complex scenario. Prog. Brain Res. 2012;197:29–51. - PubMed
-
- Sartor F., Anderson J., McCaig C., Miedzybrodzka Z., Müller B. Mutation of genes controlling mRNA metabolism and protein synthesis predisposes to neurodevelopmental disorders. Biochem. Soc. Trans. 2015;43:1259–1265. - PubMed
-
- Oberlé I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., Boué J., Bertheas M.F., Mandel J.L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

