Effect of the miR-96-5p inhibitor and mimic on the migration and invasion of the SW480-7 colorectal cancer cell line
- PMID: 31423265
- PMCID: PMC6607361
- DOI: 10.3892/ol.2019.10492
Effect of the miR-96-5p inhibitor and mimic on the migration and invasion of the SW480-7 colorectal cancer cell line
Abstract
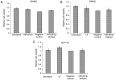
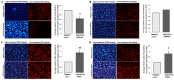
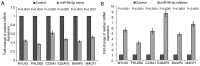
The objectives of the present study were to identify the aberrant expression of microRNA (miRNA) in colorectal carcinoma (CRC) tissues from published miRNA profiling studies and to investigate the effects of the identified miRNA inhibitor and mimic miR-96-5p on CRC cell migration and invasion. The altered expression of the regulators of cytoskeleton mRNA in miR-96-5p inhibitor-transfected cells was determined. The miR-96-5p expression level in five CRC cell lines, HCT11, CaCo2, HT29, SW480 and SW620, and 26 archived paraffin-embedded CRC tissues were also investigated by reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR). Cell viability in response to the miR-96-5p inhibitor and mimic transfections was determined by an MTT assay. A Matrigel invasion assay was conducted to select the invasive subpopulation designated SW480-7, by using the parental cell line SW480. The effects of miR-96-5p mimic- or inhibitor-transfected SW480-7 cells on cell migration and invasion were evaluated using the Transwell and Matrigel assays, and the change in expression of the regulators of cytoskeleton mRNAs was identified by Cytoskeleton Regulators RT2-Profiler PCR array followed by validation with RT-qPCR. CRC tissues exhibited a significant increase in miR-96-5p expression, compared with their matched normal adjacent tissues, indicating an oncogenic role for miR-96-5p. The results demonstrated that the miR-96-5p inhibitor decreased the migration of SW480-7 cells, but had no effect on invasion. This may be due to the promotion of cell invasion by Matrigel, which counteracts the blockade of cell invasion by the miR-96-5p inhibitor. The miR-96-5p mimic enhanced SW480-7 cell migration and invasion, as expected. It was determined that there was a >2.5 fold increase in the expression of genes involved in cytoskeleton regulation, myosin light chain kinase 2, pleckstrin homology like domain family B member 2, cyclin A1, IQ motif containing GTPase activating protein 2, Brain-specific angiogenesisinhibitor 1-associated protein 2 and microtubule-actin crosslinking factor 1, in miR-96-5p inhibitor-transfected cells, indicating that they are negative regulators of cell migration. In conclusion, the miR-96-5p inhibitor blocked cell migration but not invasion, and the latter may be due to the counteraction of Matrigel, which has been demonstrated to stimulate cell invasion.
Keywords: Matrigel; colorectal cancer; cytoskeleton; invasion; microRNA-96-5p; migration.
Figures


Similar articles
-
Inhibition of cell migration and invasion by miR‑29a‑3p in a colorectal cancer cell line through suppression of CDC42BPA mRNA expression.Oncol Rep. 2017 Dec;38(6):3554-3566. doi: 10.3892/or.2017.6037. Epub 2017 Oct 16. Oncol Rep. 2017. PMID: 29039592
-
GDPD5, a target of miR-195-5p, is associated with metastasis and chemoresistance in colorectal cancer.Biomed Pharmacother. 2018 May;101:945-952. doi: 10.1016/j.biopha.2018.03.028. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29635904
-
The role of miR-766-5p in cell migration and invasion in colorectal cancer.Exp Ther Med. 2018 Mar;15(3):2569-2574. doi: 10.3892/etm.2018.5716. Epub 2018 Jan 8. Exp Ther Med. 2018. PMID: 29456660 Free PMC article.
-
miR-6716-5p promotes metastasis of colorectal cancer through downregulating NAT10 expression.Cancer Manag Res. 2019 Jun 6;11:5317-5332. doi: 10.2147/CMAR.S197733. eCollection 2019. Cancer Manag Res. 2019. PMID: 31239781 Free PMC article.
-
MicroRNA-493-5p promotes apoptosis and suppresses proliferation and invasion in liver cancer cells by targeting VAMP2.Int J Mol Med. 2018 Mar;41(3):1740-1748. doi: 10.3892/ijmm.2018.3358. Epub 2018 Jan 2. Int J Mol Med. 2018. PMID: 29328362
Cited by
-
LIM-domain binding protein 2 was down-regulated by miRNA-96-5p inhibited the proliferation, invasion and metastasis of lung cancer H1299 cells.Clinics (Sao Paulo). 2022 Dec 3;78:100145. doi: 10.1016/j.clinsp.2022.100145. eCollection 2023. Clinics (Sao Paulo). 2022. PMID: 36473369 Free PMC article.
-
Intrathecal Injection of the Secretome from ALS Motor Neurons Regulated for miR-124 Expression Prevents Disease Outcomes in SOD1-G93A Mice.Biomedicines. 2022 Aug 29;10(9):2120. doi: 10.3390/biomedicines10092120. Biomedicines. 2022. PMID: 36140218 Free PMC article.
-
miR-96-5p Suppresses the Progression of Nasopharyngeal Carcinoma by Targeting CDK1.Onco Targets Ther. 2020 Jul 30;13:7467-7477. doi: 10.2147/OTT.S248338. eCollection 2020. Onco Targets Ther. 2020. Retraction in: Onco Targets Ther. 2022 Nov 22;15:1407-1408. doi: 10.2147/OTT.S398523. PMID: 32801769 Free PMC article. Retracted.
-
Overexpression of PAX8-AS1 Inhibits Malignant Phenotypes of Papillary Thyroid Carcinoma Cells via miR-96-5p/PKN2 Axis.Int J Endocrinol. 2021 Oct 26;2021:5499963. doi: 10.1155/2021/5499963. eCollection 2021. Int J Endocrinol. 2021. PMID: 34745257 Free PMC article.
-
microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2-FTO-m6A/MYC axis.J Exp Clin Cancer Res. 2020 Nov 12;39(1):240. doi: 10.1186/s13046-020-01731-7. J Exp Clin Cancer Res. 2020. PMID: 33183350 Free PMC article.
References
-
- Valeri N, Braconi C, Gasparini P, Murgia C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat F, et al. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell. 2014;25:469–483. doi: 10.1016/j.ccr.2014.03.006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
