Superior HbA1c control with the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with a maximum dose of 50 units of insulin degludec in Japanese individuals with type 2 diabetes in a phase 3, double-blind, randomized trial
- PMID: 31423685
- PMCID: PMC6900157
- DOI: 10.1111/dom.13859
Superior HbA1c control with the fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with a maximum dose of 50 units of insulin degludec in Japanese individuals with type 2 diabetes in a phase 3, double-blind, randomized trial
Abstract
Aims: To investigate the efficacy and safety of insulin degludec/liraglutide (IDegLira) compared with 50 U insulin degludec (degludec) or less in Japanese individuals with type 2 diabetes (T2D).
Materials and methods: In this 26-week, double-blind, multicentre, treat-to-target trial, Japanese individuals with T2D that was uncontrolled with basal or pre-mix insulin (20-50 units) were randomized (1:1) to receive IDegLira or degludec, both with metformin. The maximum dose was 50 dose steps (IDegLira) or 50 units (degludec). The primary endpoint was change from baseline in HbA1c with IDegLira vs degludec after 26 weeks of treatment.
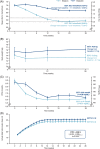
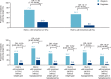
Results: In total, 210 Japanese individuals were randomized to IDegLira or degludec and completion rates were 100% and 93%, respectively. IDegLira was superior to degludec with respect to change from baseline in HbA1c: estimated treatment difference (ETD) (95% confidence interval), -13.98 mmol/Mol (-16.41; -11.55); P < 0.0001. The change in mean HbA1c was from 70.6 by -21.3 mmol/Mol with IDegLira and from 70.1 by -7.1 mmol/Mol with degludec. Mean change in body weight was -0.7 kg with IDegLira and 0.7 kg with degludec: ETD (95% CI) -1.41 kg (-2.26; -0.56); P = 0.0012. Mean daily total insulin dose was significantly lower with IDegLira (37.6 U) as compared to that with degludec (41.2 U) at Week 26. Overall rates of severe or blood glucose-confirmed hypoglycaemia and adverse events were comparable between treatment groups.
Conclusions: IDegLira provided superior reductions in HbA1c compared with ≤50 U degludec, with weight loss and similar hypoglycaemia rates and no unexpected safety or tolerability issues. These results suggest that this treatment could be an attractive intensification option for Japanese subjects with T2D that was uncontrolled with basal or pre-mixed insulin.
Keywords: basal insulin; hypoglycaemia; liraglutide; randomized trial; type 2 diabetes.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
H. W. has acted as an advisory board member for Novo Nordisk and as a speaker for Astellas Pharma, Sanofi, Mitsubishi Tanabe Pharma, Novo Nordisk, Kowa Pharmaceutical, AstraZeneca, Takeda Pharmaceutical, Novartis, Nippon Boehringer Ingelheim, Merck Sharp & Dohme, Sumitomo Dainippon Pharma, Eli Lilly Japan, Sanwa Kagaku Kenkyusho, Ono Pharmaceutical, Kissei Pharmaceutical and Fujifilm Pharma; and has received grants from Astellas Pharma, Sanofi, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, AstraZeneca, Takeda Pharmaceutical, Novartis Pharma, Nippon Boehringer Ingelheim, Merck Sharp & Dohme, Sumitomo Dainippon Pharma, Eli Lilly Japan, Ono Pharmaceutical, Kyowa Hakko Kirin, Daiichi Sankyo, Terumo, Pfizer Japan, Mochida Pharmaceutical, Taisho Toyama Pharmaceutical, Johnson & Johnson and Kowa.
S. K. has received honoraria for lectures from Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., AstraZeneca K.K., Takeda Pharmaceutical Company Limited and Mitsubishi Tanabe Pharma Corporation.
M. K. has received research support from Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., Msd K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co. Ltd., Teijin Pharma Limited, Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Daiichi Sankyo Company, Limited, Fujifilm Pharma Co., Ltd., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K. and Kowa Pharmaceutical Co. Ltd; and has participated in speakers' bureaus for Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., Msd K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Teijin Pharma Limited, Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Terumo Corporation, Daiichi Sankyo Company, Limited, Fujifilm Pharma Co., Ltd., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Medtronic Japan Co., Ltd., Maruho Co. Ltd. and Kowa Pharmaceutical Co. Ltd.
B. R. A., T. N. and M. R. are employees of and shareholders in Novo Nordisk.
J. N has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical. Co., Ltd., Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical. Co., Ltd., Sanofi K.K., Takeda Pharmaceutical. Co., Ltd., Mitsubishi Tanabe Pharma Corp., Terumo Co., Boehringer Ingelheim Japan Co. Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., research grants from Kissei Pharmaceutical. Co., Ltd., Boehringer Ingelheim Japan Co. Ltd. and EPS Co. Ltd.; and has received other honoraria from Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Daiichi Sankyo Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Japan Tobacco Inc., Novartis Pharma K.K., Novo Nordisk Pharma Ltd.
Figures

References
-
- Federation ID . IDF Atlas, 8th Edition: Japan country report. http://reports.instantatlas.com/report/view/846e76122b5f476fa6ef09471965.... Accessed March 4, 2019.
-
- Morimoto A, Nishimura R, Tamija N. Trends in the epidemiology of patients with diabetes in Japan. Japan Med Assoc J. 2010;53:36‐40.
-
- Kawaski EMN, Eguchi K. Type 1 diabetes in Japan. Diabetologia. 2006;49:828‐836. - PubMed
-
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129‐2140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

