Effects of the ventilatory stimulant, doxapram on human TASK-3 (KCNK9, K2P9.1) channels and TASK-1 (KCNK3, K2P3.1) channels
- PMID: 31423744
- PMCID: PMC7003846
- DOI: 10.1111/apha.13361
Effects of the ventilatory stimulant, doxapram on human TASK-3 (KCNK9, K2P9.1) channels and TASK-1 (KCNK3, K2P3.1) channels
Abstract
Aims: The mode of action by which doxapram acts as a respiratory stimulant in humans is controversial. Studies in rodent models, have shown that doxapram is a more potent and selective inhibitor of TASK-1 and TASK-1/TASK-3 heterodimer channels, than TASK-3. Here we investigate the direct effect of doxapram and chirally separated, individual positive and negative enantiomers of the compound, on both human and mouse, homodimeric and heterodimeric variants of TASK-1 and TASK-3.
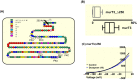
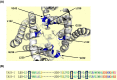
Methods: Whole-cell patch clamp electrophysiology on tsA201 cells was used to assess the potency of doxapram on cloned human or mouse TASK-1, TASK-3 and TASK-2 channels. Mutations of amino acids in the pore-lining region of TASK-3 channels were introduced using site-directed mutagenesis.
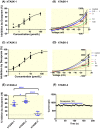
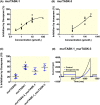
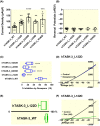
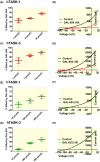
Results: Doxapram was an equipotent inhibitor of human TASK-1 and TASK-3 channels, compared with mouse channel variants, where it was more selective for TASK-1 and heterodimers of TASK-1 and TASK-3. The effect of doxapram could be attenuated by either the removal of the C-terminus of human TASK-3 channels or mutations of particular hydrophobic residues in the pore-lining region. These mutations, however, did not alter the effect of a known extracellular inhibitor of TASK-3, zinc. The positive enantiomer of doxapram, GAL-054, was a more potent antagonist of TASK channels, than doxapram, whereas the negative enantiomer, GAL-053, had little inhibitory effect.
Conclusion: These data show that in contrast to rodent channels, doxapram is a potent inhibitor of both TASK-1 and TASK-3 human channels, providing further understanding of the pharmacological profile of doxapram in humans and informing the development of new therapeutic agents.
Keywords: K2P channels; TASK-1 channels; TASK-3 channels; doxapram; enantiomers; heterodimers; respiratory stimulant.
© 2019 The Authors. Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest. We confirm that the material submitted conforms to Good Publishing Practice in Physiology: Good publication practice in physiology.59
Figures

Similar articles
-
The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration.Anesth Analg. 2006 Mar;102(3):779-85. doi: 10.1213/01.ane.0000194289.34345.63. Anesth Analg. 2006. PMID: 16492828
-
TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats.Anesth Analg. 2013 Apr;116(4):810-6. doi: 10.1213/ANE.0b013e318284469d. Epub 2013 Mar 4. Anesth Analg. 2013. PMID: 23460565 Free PMC article.
-
Breathing Stimulant Compounds Inhibit TASK-3 Potassium Channel Function Likely by Binding at a Common Site in the Channel Pore.Mol Pharmacol. 2015 Nov;88(5):926-34. doi: 10.1124/mol.115.100107. Epub 2015 Aug 12. Mol Pharmacol. 2015. PMID: 26268529 Free PMC article.
-
A new look at the respiratory stimulant doxapram.CNS Drug Rev. 2006 Fall-Winter;12(3-4):236-49. doi: 10.1111/j.1527-3458.2006.00236.x. CNS Drug Rev. 2006. PMID: 17227289 Free PMC article. Review.
-
Two-Pore-Domain Potassium (K2P-) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes.Cells. 2021 Oct 27;10(11):2914. doi: 10.3390/cells10112914. Cells. 2021. PMID: 34831137 Free PMC article. Review.
Cited by
-
Countering opioid-induced respiratory depression by non-opioids that are respiratory stimulants.F1000Res. 2020 Feb 7;9:F1000 Faculty Rev-91. doi: 10.12688/f1000research.21738.1. eCollection 2020. F1000Res. 2020. PMID: 32089833 Free PMC article. Review.
-
Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option.Front Pharmacol. 2021 Mar 4;12:638445. doi: 10.3389/fphar.2021.638445. eCollection 2021. Front Pharmacol. 2021. PMID: 33897427 Free PMC article. Review.
-
The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model.NeuroSci. 2022 Oct 23;3(4):566-588. doi: 10.3390/neurosci3040041. eCollection 2022 Dec. NeuroSci. 2022. PMID: 39483770 Free PMC article.
-
The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model.Front Physiol. 2021 Jan 21;11:629421. doi: 10.3389/fphys.2020.629421. eCollection 2020. Front Physiol. 2021. PMID: 33551849 Free PMC article.
-
Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P).Int J Mol Sci. 2022 Dec 13;23(24):15787. doi: 10.3390/ijms232415787. Int J Mol Sci. 2022. PMID: 36555429 Free PMC article.
References
-
- Lunsford CD, Cale AD Jr, Ward JW, Franko BV, Jenkins H. 4‐(β‐Substituted ethyl)‐3,3‐diphenyl‐2‐pyrrolidinones. A new series of CNS stimulants. J Med Chem. 1964;7(3):302‐310. - PubMed
-
- Winnie AP, Collins VJ. The search for a pharmacologic ventilator. Acta Anaesthesiol Scand. 1966;10(s23):63‐71. - PubMed
-
- Nishino T, Mokashi A, Lahiri S. Stimulation of carotid chemoreceptors and ventilation by doxapram in the cat. J Appl Physiol. 1982;52(5):1261‐1265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

