Sensitive and Specific Detection of Low-Level Antibody Responses in Mild Middle East Respiratory Syndrome Coronavirus Infections
- PMID: 31423970
- PMCID: PMC6759241
- DOI: 10.3201/eid2510.190051
Sensitive and Specific Detection of Low-Level Antibody Responses in Mild Middle East Respiratory Syndrome Coronavirus Infections
Abstract
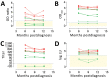
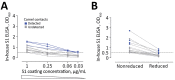
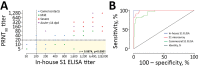
Middle East respiratory syndrome coronavirus (MERS-CoV) infections in humans can cause asymptomatic to fatal lower respiratory lung disease. Despite posing a probable risk for virus transmission, asymptomatic to mild infections can go unnoticed; a lack of seroconversion among some PCR-confirmed cases has been reported. We found that a MERS-CoV spike S1 protein-based ELISA, routinely used in surveillance studies, showed low sensitivity in detecting infections among PCR-confirmed patients with mild clinical symptoms and cross-reactivity of human coronavirus OC43-positive serum samples. Using in-house S1 ELISA and protein microarray, we demonstrate that most PCR-confirmed MERS-CoV case-patients with mild infections seroconverted; nonetheless, some of these samples did not have detectable levels of virus-neutralizing antibodies. The use of a sensitive and specific serologic S1-based assay can be instrumental in the accurate estimation of MERS-CoV prevalence.
Keywords: ELISA; MERS; MERS-CoV; Middle East respiratory syndrome coronavirus; Qatar; S1; South Korea; antibodies; camels; coronavirus; diagnostics; human; neutralizing; serology; spike; the Netherlands; viruses.
Figures


References
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) [cited 2017 Jul 16]. http://www.who.int/emergencies/mers-cov
-
- Puzelli S, Azzi A, Santini MG, Di Martino A, Facchini M, Castrucci MR, et al. Investigation of an imported case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Florence, Italy, May to June 2013. Euro Surveill. 2013;18:20564. 10.2807/1560-7917.ES2013.18.34.20564 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

