Nucleic acid and antigen detection tests for leptospirosis
- PMID: 31425612
- PMCID: PMC6699653
- DOI: 10.1002/14651858.CD011871.pub2
Nucleic acid and antigen detection tests for leptospirosis
Abstract
Background: Early diagnosis of leptospirosis may contribute to the effectiveness of antimicrobial therapy and early outbreak recognition. Nucleic acid and antigen detection tests have the potential for early diagnosis of leptospirosis. With this systematic review, we assessed the sensitivity and specificity of nucleic acid and antigen detection tests.
Objectives: To determine the diagnostic test accuracy of nucleic acid and antigen detection tests for the diagnosis of human symptomatic leptospirosis.
Search methods: We searched electronic databases including MEDLINE, Embase, the Cochrane Library, and regional databases from inception to 6 July 2018. We did not apply restrictions to language or time of publication.
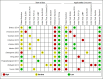
Selection criteria: We included diagnostic cross-sectional studies and case-control studies of tests that made use of nucleic acid and antigen detection methods in people suspected of systemic leptospirosis. As reference standards, we considered the microscopic agglutination test alone (which detects antibodies against leptospirosis) or in a composite reference standard with culturing or other serological tests. Studies were excluded when the controls were healthy individuals or when there were insufficient data to calculate sensitivity and specificity.
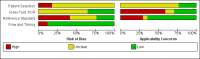
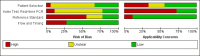
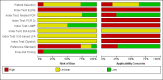
Data collection and analysis: At least two review authors independently extracted data from each study. We used the revised Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2) to assess risk of bias. We calculated study-specific values for sensitivity and specificity with 95% confidence intervals (CI) and pooled the results in a meta-analysis when appropriate. We used the bivariate model for index tests with one positivity threshold, and we used the hierarchical summary receiver operating characteristic model for index tests with multiple positivity thresholds. As possible sources of heterogeneity, we explored: timing of index test, disease prevalence, blood sample type, primers or target genes, and the real-time polymerase chain reaction (PCR) visualisation method. These were added as covariates to the meta-regression models.
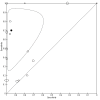
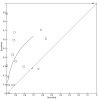
Main results: We included 41 studies evaluating nine index tests (conventional PCR (in short: PCR), real-time PCR, nested PCR, PCR performed twice, loop-mediated isothermal amplification, enzyme-linked immunosorbent assay (ELISA), dot-ELISA, immunochromatography-based lateral flow assay, and dipstick assay) with 5981 participants (1834 with and 4147 without leptospirosis). Methodological quality criteria were often not reported, and the risk of bias of the reference standard was generally considered high. The applicability of findings was limited by the frequent use of frozen samples. We conducted meta-analyses for the PCR and the real-time PCR on blood products.The pooled sensitivity of the PCR was 70% (95% CI 37% to 90%) and the pooled specificity was 95% (95% CI 75% to 99%). When studies with a high risk of bias in the reference standard domain were excluded, the pooled sensitivity was 87% (95% CI 44% to 98%) and the pooled specificity was 97% (95% CI 60% to 100%). For the real-time PCR, we estimated a summary receiver operating characteristic curve. To illustrate, a point on the curve with 85% specificity had a sensitivity of 49% (95% CI 30% to 68%). Likewise, at 90% specificity, sensitivity was 40% (95% CI 24% to 59%) and at 95% specificity, sensitivity was 29% (95% CI 15% to 49%). The median specificity of real-time PCR on blood products was 92%. We did not formally compare the diagnostic test accuracy of PCR and real-time PCR, as direct comparison studies were lacking. Three of 15 studies analysing PCR on blood products reported the timing of sample collection in the studies included in the meta-analyses (range 1 to 7 days postonset of symptoms), and nine out of 16 studies analysing real-time PCR on blood products (range 1 to 19 days postonset of symptoms). In PCR studies, specificity was lower in settings with high leptospirosis prevalence. Other investigations of heterogeneity did not identify statistically significant associations. Two studies suggested that PCR and real-time PCR may be more sensitive on blood samples collected early in the disease stage. Results of other index tests were described narratively.
Authors' conclusions: The validity of review findings are limited and should be interpreted with caution. There is a substantial between-study variability in the accuracy of PCR and real-time PCR, as well as a substantial variability in the prevalence of leptospirosis. Consequently, the position of PCR and real-time PCR in the clinical pathway depends on regional considerations such as disease prevalence, factors that are likely to influence accuracy, and downstream consequences of test results. There is insufficient evidence to conclude which of the nucleic acid and antigen detection tests is the most accurate. There is preliminary evidence that PCR and real-time PCR are more sensitive on blood samples collected early in the disease stage, but this needs to be confirmed in future studies.
Conflict of interest statement
Conflict of interest
The Leptospirosis Reference Center (AMC), formerly part of the Royal Tropical Institute (KIT), has done multiple diagnostic accuracy studies. Three such studies are included in this review (Gravekamp 1993; Ahmed 2009; Denipitiya 2016).
Financial conflicts
BY: none. SGV: none. AA: none. BJV: none. IN: none. RS: none. MPG: none. RH: none. MG: none. ML: none.
Figures



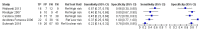


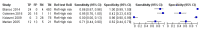

Update of
- doi: 10.1002/14651858.CD011871
Similar articles
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
Diagnosis of human leptospirosis: systematic review and meta-analysis of the diagnostic accuracy of the Leptospira microscopic agglutination test, PCR targeting Lfb1, and IgM ELISA to Leptospira fainei serovar Hurstbridge.BMC Infect Dis. 2024 Feb 7;24(1):168. doi: 10.1186/s12879-023-08935-0. BMC Infect Dis. 2024. PMID: 38326762 Free PMC article.
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2020 Aug 26;8(8):CD013705. doi: 10.1002/14651858.CD013705. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 24;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. PMID: 32845525 Free PMC article. Updated.
-
Rapid diagnostic tests for plague.Cochrane Database Syst Rev. 2020 Jun 26;6(6):CD013459. doi: 10.1002/14651858.CD013459.pub2. Cochrane Database Syst Rev. 2020. PMID: 32597510 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD013639. doi: 10.1002/14651858.CD013639.pub4. PMID: 33242342 Updated.
Cited by
-
Case Report: Leptospirosis Complicated by Persistent, Bilateral Sensorineural Hearing Loss.Am J Trop Med Hyg. 2023 Oct 9;109(6):1238-1241. doi: 10.4269/ajtmh.23-0100. Print 2023 Dec 6. Am J Trop Med Hyg. 2023. PMID: 37962328 Free PMC article.
-
Role of Diagnostics in Epidemiology, Management, Surveillance, and Control of Leptospirosis.Pathogens. 2022 Mar 24;11(4):395. doi: 10.3390/pathogens11040395. Pathogens. 2022. PMID: 35456070 Free PMC article. Review.
-
Misdiagnosis of pneumorrhagia leptospirosis as common CAP: A case report and literature review.Respir Med Case Rep. 2023 Nov 27;46:101954. doi: 10.1016/j.rmcr.2023.101954. eCollection 2023. Respir Med Case Rep. 2023. PMID: 38094659 Free PMC article.
-
Diagnostic Advances in Leptospirosis: A Comparative Analysis of Paraclinical Tests with a Focus on PCR.Microorganisms. 2025 Mar 15;13(3):667. doi: 10.3390/microorganisms13030667. Microorganisms. 2025. PMID: 40142559 Free PMC article. Review.
-
Culture-Independent Detection and Identification of Leptospira Serovars.Microbiol Spectr. 2022 Dec 21;10(6):e0247522. doi: 10.1128/spectrum.02475-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445143 Free PMC article.
References
References to studies included in this review
Agampodi 2012 {published data only}
Agampodi 2016 {published data only}
Ahmed 2009 {published data only}
Ananyina 2000 {published data only}
-
- Ananyina YV, Samsonova AP, Lebedev VV, Petrov EM, Esipov EN. Gene diagnostics of acute persistent Leptospira infection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2000;4(Suppl):23‐6. - PubMed
Backstedt 2015 {published data only}
Biscornet 2017 {published data only}
Blanco 2014 {published data only}
-
- Blanco RM, Romero EC. Evaluation of nested polymerase chain reaction for the early detection of Leptospira spp. DNA in serum samples from patients with leptospirosis. Diagnostic Microbiology & Infectious Disease 2014;78(4):343‐6. - PubMed
Cardona 2008 {published data only}
-
- Cardona MN, Moros RM, López EA. Diagnosis of leptospirosis by PCR in patients with febrile icterohemorrhagic syndrome [Diagnóstico de leptospirosis mediante la PCR en pacientes con síndrome febril icterohemorrágico]. Revista de la Sociedad Venezolana de Microbiología 2008;28(1):24‐30.
Céspedes 2007 {published data only}
-
- Céspedes M, Tapia R, Balda L, Gonzalez D, Peralta C, Condori P. Standardization and validation of a PCR test for early human leptospirosis [Estandarización y validación de una prueba de PCR para el diagnóstico precoz de Leptospirosis humana]. Revista Peruana de Medicina Experimental y Salud Pública 2007;24(1):20‐6.
Chandrasiri 2010 {unpublished data only}
-
- Chandrasiri P, Wahala P, Ramesh R, Kulathunge K. Detection of Leptospira by PCR and comparison of different methods for early diagnosis of leptospirosis. Clinical Microbiology and Infection 2010;16:S394.
Chaurasia 2018 {published data only}
-
- Chaurasia R, Thresiamma KC, Eapen CK, Zachariah BJ, Paul R, Sritharan M. Pathogen‐specific leptospiral proteins in urine of patients with febrile illness aids in differential diagnosis of leptospirosis from dengue. European Journal of Clinical Microbiology & Infectious Diseases 2018;37(3):423‐33. - PubMed
de Abreu Fonseca 2006 {published data only}
-
- Abreu Fonseca C, Teixeira de Freitas VL, Calo Romero E, Spinosa C, Arroyo Sanches MC, Silva MV, et al. Polymerase chain reaction in comparison with serological tests for early diagnosis of human leptospirosis. Tropical Medicine & International Health 2006;11(11):1699‐707. - PubMed
Denipitiya 2016 {published data only}
-
- Denipitiya DT, Chandrasekharan NV, Abeyewickreme W, Hartskeerl CM, Hartskeerl RA, Jiffrey AM, et al. Application of a real time polymerase chain reaction (PCR) assay for the early diagnosis of human leptospirosis in Sri Lanka. Biologicals 2016;44(6):497‐502. - PubMed
Fan 1999 {published data only}
-
- Fan Q, Cao D, Liu J. Rapid detection of Leptospira DNA by polymerase chain reaction. en.cnki.com.cn/Article_en/CJFDTOTAL‐ZRSZ902.025.htm 1999 (accessed 3 August 2017).
Gokmen 2016 {published data only}
Gonzalez 2013 {published data only}
-
- Gonzalez S, Geymonat JP, Hernandez E, Marques JM, Schelotto F, Varela G. Usefulness of real‐time PCR assay targeting lipL32 gene for diagnosis of human leptospirosis in Uruguay. Journal of Infection in Developing Countries 2013;7(12):941‐5. - PubMed
Gravekamp 1993 {published data only}
-
- Gravekamp C, Kemp H, Franzen M, Carrington D, Schoone GJ, Eys GJ, et al. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. Journal of General Microbiology 1993;139(8):1691‐700. - PubMed
Kitashoji 2015 {published data only}
Koizumi 2009 {published data only}
-
- Koizumi N, Gamage CD, Muto M, Kularatne SA, Budagoda BD, Rajapakse RP, et al. Serological and genetic analysis of leptospirosis in patients with acute febrile illness in Kandy, Sri Lanka. Japanese Journal of Infectious Diseases 2009;62(6):474‐5. - PubMed
Merien 2005 {published data only}
-
- Merien F, Portnoi D, Bourhy P, Charavay F, Berlioz‐Arthaud A, Baranton G. A rapid and quantitative method for the detection of Leptospira species in human leptospirosis. FEMS Microbiology Letters 2005;249(1):139‐47. - PubMed
Ooteman 2006 {published data only}
-
- Ooteman MC, Vago AR, Koury MC. Evaluation of MAT, IgM ELISA and PCR methods for the diagnosis of human leptospirosis. Journal of Microbiological Methods 2006;65(2):247‐57. - PubMed
Pakoa 2018 {published data only}
-
- Pakoa JG, Soupe‐Gilbert ME, Girault D, Takau D, Gaviga J, Gourinat AC, et al. High incidence of leptospirosis in an observational study of hospital outpatients in Vanuatu highlights the need for improved awareness and diagnostic capacities. PLoS Neglected Tropical Diseases 2018;12(6):e0006564. - PMC - PubMed
Riediger 2007 {published data only}
-
- Riediger IN, Moreirab SD, Skrabaa I, Leônidas J, Hoffmannc LU, Nakatania SM, et al. Use of Urine and Blood Samples for PCR Diagnosis of Human Leptospirosis [Dissertation]. Paraná (Brazil): Federal University of Paraná, 2007.
Riediger 2017 {published data only}
Saengjaruk 2002 {published data only}
Samsonova 1997 {published data only}
-
- Samsonova AP, Chzhun‐Fu L, Petrov EM, Aliapkina IS, Epon L, Gintsburg AL, et al. Development of polymerase chain reaction‐based test systems for detecting leptospira in polytypical leptospirosis foci. Molekuliarnaia Genetika, Mikrobiologiia i Virusologiia 1997;4:15‐9. - PubMed
Seng 2007 {published data only}
-
- Seng H, Sok T, Tangkanakul W, Petkanchanapong W, Kositanont U, Sareth H, et al. Leptospirosis in Takeo Province, Kingdom of Cambodia, 2003. Journal of the Medical Association of Thailand 2007;90(3):546‐51. - PubMed
Sonthayanon 2013 {published data only}
-
- Sonthayanon P, Chierakul W, Wuthiekanun V, Limmathurotsakul D, Amornchai P, Smythe LD, et al. Molecular confirmation of co‐infection by pathogenic Leptospira spp. and Orientia tsutsugamushi in patients with acute febrile illness in Thailand. American Journal of Tropical Medicine & Hygiene 2013;89(4):797‐9. - PMC - PubMed
Sukmark 2018 {published data only}
-
- Sukmark T, Lumlertgul N, Peerapornratana S, Khositrangsikun K, Tungsanga K, Sitprija V, et al. Thai‐Lepto‐on‐admission probability (THAI‐LEPTO) score as an early tool for initial diagnosis of leptospirosis: result from Thai‐Lepto AKI study group. PLoS Neglected Tropical Diseases 2018;12(3):e0006319. - PMC - PubMed
Thaipadungpanit 2011 {published data only}
Vanasco 2016 {published data only}
-
- Vanasco NB, Jacob P, Landolt N, Chiani Y, Schmeling MF, Cudos C, et al. Diagnostic accuracy of an IgM enzyme‐linked immunosorbent assay and comparison with 2 polymerase chain reactions for early diagnosis of human leptospirosis. Diagnostic Microbiology and Infectious Disease 2016;84:292‐7. - PubMed
Villumsen 2012 BC {published data only}
-
- Villumsen S, Pedersen R, Borre MB, Ahrens P, Jensen JS, Krogfelt KA. Novel TaqMan PCR for detection of Leptospira species in urine and blood: pit‐falls of in silico validation. Journal of Microbiological Methods 2012;91(1):184‐90. - PubMed
Villumsen 2012 U {published data only}
-
- Villumsen S, Pedersen R, Borre MB, Ahrens P, Jensen JS, Krogfelt KA. Novel TaqMan PCR for detection of Leptospira species in urine and blood: pit‐falls of in silico validation. Journal of Microbiological Methods 2012;91(1):184‐90. - PubMed
Waggoner 2014 {published data only}
Waggoner 2015 {published data only}
Wangroongsarb 2005 {published data only}
-
- Wangroongsarb P, Yasaeng S, Petkanchanapong W, Naigowit P, Hagiwara T, Kawabata H, et al. Applicability of polymerase chain reaction to diagnosis of leptospirosis. Journal of Tropical Medicine and Parasitology 2005;28(2):43‐7.
Widiyanti 2013 {published data only}
Woods 2018 {published data only}
Wu 1996 {published data only}
-
- Weihua W, Shengfu L, Baomin D. Rapid treatment and PCR detection of serum samples from patients with leptospirosis. www.rsghb.cn/CN/abstract/article_19932.shtml 1996 (accessed 3 August 2017).
Yersin 1998 {published data only}
-
- Yersin C, Bovet P, Merien F, Wong T, Panowsky J, Perolat P. Human leptospirosis in the Seychelles (Indian Ocean): a population‐based study. American Journal of Tropical Medicine & Hygiene 1998;59(6):933‐40. - PubMed
Zhang 1992 {published data only}
-
- Zhang Y, Dai B. Detection of leptospiral DNA in the serum of 175 patients with early leptospirosis by polymerase chain reaction. Hua Xi Yi Ke da Xue Xue Bao [Journal of West China University of Medical Sciences] 1992;23:256‐60. - PubMed
References to studies excluded from this review
Ananyina 1999 {published data only}
-
- Ananyina YV, Samsonova AP. Diagnostic efficacy of the polymerase chain reaction at different stages of leptospira infection. Klinicheskaia Laboratornaia Diagnostika 1999;11:10.
Bal 1994 {published data only}
Barreto 2000 {unpublished data only}
-
- Barreto IM, Bernardo CCM, Pregnolatto BP, Romero EC. Comparison of polymerase chain reaction with microscopic agglutination test for diagnosis of human leptospirosis. Abstracts of the General Meeting of the American Society for Microbiology 2000;100:171.
Brown 1995 {published data only}
-
- Brown PD, Gravekamp C, Carrington DG, Kemp H, Hartskeerl RA, Edwards CN, et al. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. Journal of Medical Microbiology 1995;43(2):110‐4. - PubMed
Calderaro 2002 {published data only}
-
- Calderaro A, Guegan R, Piccolo G, Ragni P, Conter M, Zuelli C, et al. Leptospira spp. research by double polymerase chain reaction (nested‐PCR) [Ricerca di Leptospira spp. mediante doppia reazione polimerasica a catena (nested‐PCR)]. Microbiologia Medica 2002;17(1):37‐42.
Capriles 2017a {published data only}
-
- Capriles S, Dos Santos C, Espino GC, Salas E, Crozzoli R, Flores E, et al. Leptospirosis: surveillance of cases of the icterohemorrhagic febrile syndrome program. Aragua, Venezuela [Leptospirosis: vigilancia de casos del programa sindrome febril icterohemorragico. Aragua, Venezuela]. Comunidad Salud 2017;15(1):9‐19.
Capriles 2017b {published data only}
-
- Capriles S, Dos Santos C, Garcia CE, Salas E, Crozzoli R, Flores E, et al. Leptospirosis: surveillance of cases in the Aragua Hemorrhagic Jaundice Febrile Syndrome Program. Comunidad Y Salud 2017;15(1):9‐19.
Cermakova 2013 {published data only (unpublished sought but not used)}
-
- Cermakova Z, Kucerova P, Valenta Z, Pliskova L, Bolehovska R, Prasil P, et al. Leptospirosis: possibilities of early laboratory and clinical diagnosis. Central European Journal of Medicine 2013;8(1):84‐9.
Chanket 2003 {published data only}
-
- Chanket T. PCR diagnosis of leptospirosis based on genes from the Rfb Locus [Masters thesis]. Mahidol University, 2003.
Chu 1998 {published data only}
-
- Chu KM, Rathinam R, Namperumalsamy P, Dean D. Identification of Leptospira species in the pathogenesis of uveitis and determination of clinical ocular characteristics in south India. Journal of Infectious Diseases 1998;177(5):1314‐21. - PubMed
Destura 2007 {published data only (unpublished sought but not used)}
-
- Destura RV, Alejandria MM, Mendoza MT, Cajucom MA, Concepcion FA. The clinical utility of monoclonal antibody based DOT‐ELISA urine antigen detection in the diagnosis of leptospirosis. Philippine Journal of Internal Medicine 2007;45:1‐6.
Dittrich 2016 {published data only (unpublished sought but not used)}
Esteves 2018 {published data only}
Fhogartaigh 2014 {unpublished data only}
-
- Fhogartaigh CN, Dittrich S, Chanthongthip A, Dance DA, Newton PN, Shetty N, et al. Comparison of two molecular diagnostic approaches using serum and buffy coat for rapid diagnosis of acute leptospirosis. American Journal of Tropical Medicine and Hygiene 2014;91(5 Suppl):317.
Gosi 2012 {unpublished data only}
-
- Gosi P, Lanteri C, Fukuda M, Jongsakul K, Tyner S, Saunders D, et al. Development and validation of a quantitative PCR (TaqMan) assay for detection and early diagnosis of leptospirosis interrogans using the Joint Biological Agent Identification and Diagnostic System (JBAIDS). American Journal of Tropical Medicine and Hygiene 2012;87(5 Suppl 1):324.
Hochedez 2013 {published data only}
-
- Hochedez P, Escher M, Decoussy H, Pasgrimaud L, Martinez R, Rosine J, et al. Outbreak of leptospirosis among canyoning participants, Martinique, 2011. Euro Surveillance 2013;18(18):20472. - PubMed
Hodge 1996 {unpublished data only}
-
- Hodge W, Sivakumar R, Dean D. Leptospirosis induced uveitis: ophthalmic characteristics and methods of diagnosis. Investigative Ophthalmology & Visual Science 1996;37(3):S361.
Iwasaki 2016 {published data only (unpublished sought but not used)}
-
- Iwasaki H, Chagan‐Yasutan H, Leano PS. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagnostic Microbiology and Infectious Disease 2016;84(4):287‐91. - PubMed
Jie 2017 {published data only}
-
- Jie WU, Dan W, Shao‐ling W, Li C, Hao‐ran YE, Zhong‐hua DU, et al. Leptospirosis detection among patients with fever in Hainan Province, China. Chinese Journal of Zoonoses 2017;33(3):276‐9.
Kucerova 2013 {published data only (unpublished sought but not used)}
-
- Kucerova P, Cermakova Z, Pliskova L, Pavlis O, Kubickova P, Kleprlikova H, et al. Our experience using real‐time PCR for the detection of the gene that encodes the superficial lipoprotein LipL32 of the pathogenic leptospires to confirm the acute form of human leptospirosis. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, Czech Republic 2013;157(4):387‐91. - PubMed
Kuntawunginn 2013 {unpublished data only}
-
- Kuntawunginn W, Gosi P, Jongsakul K, Tyner S, Arsanok M, Assawariyathipat T, et al. Development and validation of a quantitative PCR (TaqMan) assay for detection of acute early cases of pathogenic leptospira species in clinical samples from Thailand. American Journal of Tropical Medicine and Hygiene 2013;11:S89.
Macak 1971 {published data only}
-
- Macak J. Diagnosis of Weil's disease using immunofluorescence technic. Ceskoslovenska Patologie 1971;7(3):154‐6. - PubMed
Merien 1995 {published data only (unpublished sought but not used)}
-
- Merien F, Baranton G, Perolat P. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. Journal of Infectious Diseases 1995;172(1):281‐5. - PubMed
Mullan 2016 {published data only}
Narayanan 2016 {published data only (unpublished sought but not used)}
-
- Narayanan R, Sumathi G, Prabhakaran SG, Shanmughapriya S, Natarajaseenivasan K. Paediatric leptospirosis: a population based case‐control study from Chennai, India. Indian Journal of Medical Microbiology 2016;34(2):228‐32. - PubMed
Natarajaseenivasan 2012 {published data only (unpublished sought but not used)}
Ooteman 2004 {published data only}
-
- Ooteman MC, Vago AR, Koury MC. Potential application of low‐stringency single specific primer‐PCR in the identification of Leptospira in the serum of patients with suspected leptospirosis. Canadian Journal of Microbiology 2004;50(12):1073‐9. - PubMed
Patil 2016 {unpublished data only}
-
- Patil DY, Dahake RV, Chowdhary AS, Deshmukh RA. Clinico‐epidemiological observations of human leptospirosis from Mumbai, India. Journal of Infection and Public Health 2016;10(2):247‐8. - PubMed
Romero 1998 {published data only}
Romero 2000 {unpublished data only}
-
- Romero EC, Barreto IM, Bernardo CC, Yasuda PH. Leptospiral meningitis diagnosed by PCR. Abstracts of the General Meeting of the American Society for Microbiology 2000;100:171‐2.
Romero 2010 {published data only}
-
- Romero EC, Blanco RM, Yasuda PH. Aseptic meningitis caused by Leptospira spp diagnosed by polymerase chain reaction. Memorias do Instituto Oswaldo Cruz 2010;105(8):988‐92. - PubMed
Samsonova 2004 {unpublished data only}
-
- Samsonova AP, Petrov EM, Lebedev VV, Lysenko IV, Alyapkina YS, Ananina YV. Genome polymorphism of pathogenic leptospires and PCR diagnosis of leptospirosis. Klinicheskaia Laboratornaia Diagnostika 2004;9:30.
Saravanan 2014 {published data only (unpublished sought but not used)}
-
- Saravanan R, Saradhai P, Rani E, Rajasekar V. A comparative study on microscopic agglutination test and counterimmunoelectrophoresis for early detection of human leptospirosis. Indian Journal of Medical Microbiology 2014;32(1):26‐30. - PubMed
Shekatkar 2010 {published data only (unpublished sought but not used)}
-
- Shekatkar S, Harish BN, Parija SC. Diagnosis of leptospirosis by polymerase chain reaction. International Journal of Pharma and Bio Sciences 2010;1(3):1‐6.
Tagoe 2011 {unpublished data only}
-
- Tagoe JA, Puplampu N, Nimo‐Paintsil SC, Kronmann KC, Clemens M, Nyarko E, et al. Leptospirosis in acute febrile patients in Ghana: diagnosis by culture, serology and polymerase chain reaction. American Journal of Tropical Medicine and Hygiene 2011;85(6 Suppl):328‐9.
Taurustiati 2013 {published data only (unpublished sought but not used)}
-
- Taurustiati D. Combination of antibody detection and antigen detection for the development of a diagnosis of leptospirosis [Kombinasi deteksi antibodi dan deteksi antigen untuk pengembangan diagnosis leptospirosis]. repository.unpad.ac.id/17506/1/pustaka_unpad_kombinasi_deteksi.pdf (accessed 3 August 2017).
Teamkrim 2005 {published data only}
-
- Teankrim S. Sensitivity and specificity of PCR for the diagnosis of leptospirosis [Dissertation]. Bangkok: Department of Medicine. Faculty of Medicine, Siriraj Hospital. Mahidol University, 2005.
Additional references
Bharti 2003
-
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infectious Diseases 2003;3:757‐71. - PubMed
Brett‐Major 2012
Budihal 2014
Costa 2003
-
- Costa E, Lopes AA, Sacramento E, Costa YA, Matos ED, Lopes MB, et al. Penicillin at the late stage of leptospirosis: a randomized controlled trial. Revista do Instituto de Medicina Tropical de São Paulo 2003;45:141‐5. - PubMed
Costa 2015
Duarte 2019
-
- Duarte JL, Giatti LL. Leptospirosis incidence in a state capital in the Western Brazilian Amazon and its relationship with climate and environmentalvariability, 2008‐2013.. Epidemiologia e Serviços de Saúde 2019;28(1):e2017224. - PubMed
Edwards 1988
-
- Edwards CN, Nicholson GD, Hassell TA, Everard CO, Callender J. Penicillin therapy in icteric leptospirosis. American Journal of Tropical Medicine and Hygiene 1988;39:388‐90. - PubMed
Farr 1995
-
- Farr RW. Leptospirosis. Clinical Infectious Diseases 1995;21:1‐6; quiz 7‐8. - PubMed
Goris 2011
-
- Goris MG, Boer KR, Bouman‐Strijker M, Hartskeerl R, Lucas C, Leeflang M. Serological laboratory tests for diagnosis of human leptospirosis in patients presenting with clinical symptoms. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009406] - DOI
Goris 2012
-
- Goris MG, Leeflang MM, Boer KR, Goeijenbier M, Gorp EC, Wagenaar JF, et al. Establishment of valid laboratory case definition for human leptospirosis. Journal of Bacteriology and Parasitology 2012;3:2.
Goris 2013
Guerra 2013
Hall 2014
-
- Hall C, Lambourne J. The challenges of diagnosing leptospirosis. Journal of Travel Medicine 2014;21(2):139‐40. - PubMed
Jensenius 2013
-
- Jensenius M, Han PV, Schlagenhauf P, Schwartz E, Parola P, Castelli F, et al. GeoSentinel Surveillance Network. Acute and potentially life‐threatening tropical diseases in western travelers – a GeoSentinel multicenter study, 1996‐2011. American Journal of Tropical Medicine and Hygiene 2013;88(2):397‐404. - PMC - PubMed
Lau 2010
-
- Lau C, Smythe L, Weinstein P. Leptospirosis: an emerging disease in travellers. Travel Medicine and Infectious Disease 2010;8(1):33‐9. - PubMed
Levett 2001
Limmathurotsakul 2012
-
- Limmathurotsakul D, Turner EL, Wuthiekanun V, Thaipadungpanit J, Suputtamongkol Y, Chierakul W, et al. Fool's gold: why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clinical Infectious Diseases 2012;55:322‐31. - PMC - PubMed
McBride 2005
-
- McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Current Opinion in Infectious Diseases 2005;18:376‐86. - PubMed
McClain 1984
-
- McClain JB, Ballou WR, Harrison SM, Steinweg DL. Doxycycline therapy for leptospirosis. Annals of Internal Medicine 1984;100:696‐8. - PubMed
Mwachui 2015
Pappas 2008
-
- Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. International Journal of Infectious Diseases 2008;12:351‐7. - PubMed
Picardeau 2014
-
- Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, Durski K, Hartskeerl RA. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagnostic Microbiology and Infectious Disease 2014;78:1‐8. - PubMed
Pijnacker 2016
-
- Pijnacker R, Goris MG, Wierik MJ, Broens EM, Giessen JW, Rosa M, et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Eurosurveillance 2016;21(17):1‐7. - PubMed
Rutjes 2005
-
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case‐control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51:1335‐41. - PubMed
Rutjes 2007
-
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. Health Technology Assessment 2007;11(50):iii, ix‐51. - PubMed
Vijayachari 2008
-
- Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. Journal of Biosciences 2008;33:557‐69. - PubMed
Warnasekara 2019
Watt 1988
-
- Watt G, Padre LP, Tuazon ML, Calubaquib C, Santiago E, Ranoa CP, et al. Placebo‐controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 1988;1:433‐5. - PubMed
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies.. Annals of Internal Medicine 2011;155(8):529‐36. - PubMed
WHO 2003
-
- World Health Organization. Human leptospirosis: guidance for diagnosis, surveillance and control, 2003. whqlibdoc.who.int/hq/2003/WHO_CDS_CSR_EPH_2002.23.pdf (accessed 3 August 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

