Interventions to increase adherence to medications for tobacco dependence
- PMID: 31425618
- PMCID: PMC6699660
- DOI: 10.1002/14651858.CD009164.pub3
Interventions to increase adherence to medications for tobacco dependence
Abstract
Background: Pharmacological treatments for tobacco dependence, such as nicotine replacement therapy (NRT), have been shown to be safe and effective interventions for smoking cessation. Higher levels of adherence to these medications increase the likelihood of sustained smoking cessation, but many smokers use them at a lower dose and for less time than is optimal. It is important to determine the effectiveness of interventions designed specifically to increase medication adherence. Such interventions may address motivation to use medication, such as influencing beliefs about the value of taking medications, or provide support to overcome problems with maintaining adherence.
Objectives: To assess the effectiveness of interventions aiming to increase adherence to medications for smoking cessation on medication adherence and smoking abstinence compared with a control group typically receiving standard care.
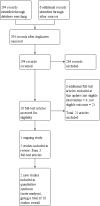
Search methods: We searched the Cochrane Tobacco Addiction Group Specialized Register, and clinical trial registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform) to the 3 September 2018. We also conducted forward and backward citation searches.
Selection criteria: Randomised, cluster-randomised or quasi-randomised studies in which adults using active pharmacological treatment for smoking cessation were allocated to an intervention arm where there was a principal focus on increasing adherence to medications for tobacco dependence, or a control arm providing standard care. Dependent on setting, standard care may have comprised minimal support or varying degrees of behavioural support. Included studies used a measure that allowed assessment of the degree of medication adherence.
Data collection and analysis: Two authors independently screened studies for eligibility, extracted data for included studies and assessed risk of bias. For continuous outcome measures, we calculated effect sizes as standardised mean differences (SMDs). For dichotomous outcome measures, we calculated effect sizes as risk ratios (RRs). In meta-analyses for adherence outcomes, we combined dichotomous and continuous data using the generic inverse variance method and reported pooled effect sizes as SMDs; for abstinence outcomes, we reported and pooled dichotomous outcomes. We obtained pooled effect sizes with 95% confidence intervals (CIs) using random-effects models. We conducted subgroup analyses to assess whether the primary focus of the adherence treatment ('practicalities' versus 'perceptions' versus both), the delivery approach (participant versus clinician-centred) or the medication type were associated with effectiveness.
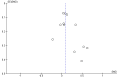
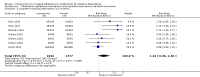
Main results: We identified two new studies, giving a total of 10 studies, involving 3655 participants. The medication adherence interventions studied were all provided in addition to standard behavioural support.They typically provided further information on the rationale for, and emphasised the importance of, adherence to medication or supported the development of strategies to overcome problems with maintaining adherence (or both). Seven studies targeted adherence to NRT, two to bupropion and one to varenicline. Most studies were judged to be at high or unclear risk of bias, with four of these studies judged at high risk of attrition or detection bias. Only one study was judged to be at low risk of bias.Meta-analysis of all 10 included studies (12 comparisons) provided moderate-certainty evidence that adherence interventions led to small improvements in adherence (i.e. the mean amount of medication consumed; SMD 0.10, 95% CI 0.03 to 0.18; I² = 6%; n = 3655), limited by risk of bias. Subgroup analyses for the primary outcome identified no significant subgroup effects, with effect sizes for subgroups imprecisely estimated. However, there was a very weak indication that interventions focused on the 'practicalities' of adhering to treatment (i.e. capabilities, resources, levels of support or skills) may be effective (SMD 0.21, 95% CI 0.03 to 0.38; I² = 39%; n = 1752), whereas interventions focused on treatment 'perceptions' (i.e. beliefs, cognitions, concerns and preferences; SMD 0.10, 95% CI -0.03 to 0.24; I² = 0%; n = 839) or on both (SMD 0.04, 95% CI -0.08 to 0.16; I² = 0%; n = 1064), may not be effective. Participant-centred interventions may be effective (SMD 0.12, 95% CI 0.02 to 0.23; I² = 20%; n = 2791), whereas those that are clinician-centred may not (SMD 0.09, 95% CI -0.05 to 0.23; I² = 0%; n = 864).Five studies assessed short-term smoking abstinence (five comparisons), while an overlapping set of five studies (seven comparisons) assessed long-term smoking abstinence of six months or more. Meta-analyses resulted in low-certainty evidence that adherence interventions may slightly increase short-term smoking cessation rates (RR 1.08, 95% CI 0.96 to 1.21; I² = 0%; n = 1795) and long-term smoking cessation rates (RR 1.16, 95% CI 0.96 to 1.40; I² = 48%; n = 3593). In both cases, the evidence was limited by risk of bias and imprecision, with CIs encompassing minimal harm as well as moderate benefit, and a high likelihood that further evidence will change the estimate of the effect. There was no evidence that interventions to increase adherence to medication led to any adverse events. Studies did not report on factors plausibly associated with increases in adherence, such as self-efficacy, understanding of and attitudes toward treatment, and motivation and intentions to quit.
Authors' conclusions: In people who are stopping smoking and receiving behavioural support, there is moderate-certainty evidence that enhanced behavioural support focusing on adherence to smoking cessation medications can modestly improve adherence. There is only low-certainty evidence that this may slightly improve the likelihood of cessation in the shorter or longer-term. Interventions to increase adherence can aim to address the practicalities of taking medication, change perceptions about medication, such as reasons to take it or concerns about doing so, or both. However, there is currently insufficient evidence to confirm which approach is more effective. There is no evidence on whether such interventions are effective for people who are stopping smoking without standard behavioural support.
Conflict of interest statement
GJH is an author of one of the studies included in the review (Marteau 2012).
FN declares no competing interests.
AF declares researcher‐led funding from Johnson and Johnson (Ethicon) being awarded to her institution.
NL is managing editor of the Cochrane Tobacco Addiction Group, although this is not deemed a conflict of interest.
PA is an author of one the studies included in the review (Marteau 2012). PA is also the co‐ordinating editor of the Cochrane Tobacco Addiction Group, although this is not deemed a conflict of interest.
Figures










Update of
-
Interventions to increase adherence to medications for tobacco dependence.Cochrane Database Syst Rev. 2015 Feb 23;(2):CD009164. doi: 10.1002/14651858.CD009164.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Aug 16;8:CD009164. doi: 10.1002/14651858.CD009164.pub3. PMID: 25914910 Updated.
References
References to studies included in this review
Chan 2010 {published data only}
-
- Chan SS, Leung DY, Abdullah AS, Lo SS, Yip AW, Kok W‐M, et al. Smoking‐cessation and adherence intervention among Chinese patients with erectile dysfunction. American Journal of Preventive Medicine 2010;39(3):251‐8. - PubMed
Chan 2011 {published data only}
-
- Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam T‐H. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction 2011;106:1155‐63. - PubMed
Marteau 2012 {published data only}
Mooney 2005 {published data only}
-
- Mooney M, Babb D, Jensen J, Hatsukami D. Interventions to increase use of nicotine gum: a randomized, controlled, single‐blind trial. Nicotine & Tobacco Research 2005;7(4):565‐79. - PubMed
-
- Mooney M, Green C, Hatsukami D. Nicotine self‐administration: cigarette versus nicotine gum diurnal topography. Human Psychopharmacology 2006;21(8):539‐48. - PubMed
Mooney 2007 {published data only}
-
- Mooney ME, Sayre SL, Hokanson PS, Stotts AL, Schmitz JM. Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: a replication and extension study. Addictive Behaviors 2007;32:875‐80. - PubMed
Nollen 2011 {published data only}
Schlam 2018 {published data only}
Schmitz 2005 {published data only}
-
- Schmitz JM, Sayre SL, Stotts AL, Rothfleisch J, Mooney ME. Medication compliance during a smoking cessation clinical trial: a brief intervention using MEMS feedback. Journal of Behavioral Medicine 2005;28(2):139‐47. - PubMed
Smith 2013 {published data only}
References to studies excluded from this review
Aveyard 2007 {published data only}
Bansal‐Travers 2010 {published data only}
Berlin 2011 {published data only}
-
- Berlin I, Jacob N, Coudert M, Perriot J, Schultz L, Rodon N. Adjustment of nicotine replacement therapies according to saliva cotinine concentration: the ADONIS* trial – a randomized study in smokers with medical comorbidities. Addiction 2011;106(4):833‐43. - PubMed
Bock 2014 {published data only}
Brendryen 2008 {published data only}
-
- Brendryen H, Kraft P. Happy ending: a randomized controlled trial of a digital multi‐media smoking cessation intervention. Addiction 2008;103:478‐84. - PubMed
Buchanan 2004 {published data only}
-
- Buchanan LM, El‐Banna M, White A, Moses S, Siedlik C, Wood M. An exploratory study of multicomponent treatment intervention for tobacco dependency. Journal of Nursing Scholarship 2004;36(4):324‐30. - PubMed
Cropsey 2017 {published data only}
-
- Cropsey KL, Hendricks PS, Schiavon S, Sellers A, Froelich M, Shelton RC, et al. A pilot trial of In vivo NRT sampling to increase medication adherence in community corrections smokers. Addictive Behaviors 2017;67:92‐9. - PubMed
Gariti 2009 {published data only}
Gong 2016 {published data only}
ICRFGPRG 1993 {published data only}
Ingersoll 2009 {published data only}
ISRCTN33423896 {published data only}
-
- ISRCTN33423896. Study of effectiveness of a smartphone app for stopping smoking focused on use of nicotine replacement therapy. isrctn.com/ISRCTN33423896 (first received 16 March 2015).
Lando 1988 {published data only}
-
- Lando HA, Kalb EA, McGovern PG. Behavioral self‐help materials as an adjunct to nicotine gum. Addictive Behaviors 1988;13(2):181‐4. - PubMed
Lifrak 1997 {published data only}
-
- Lifrak P, Gariti P, Alterman A, McKay J, Volpicelli J, Sparkman T, et al. Results of two levels of adjunctive treatment used with the nicotine patch. American Journal on Addictions 1997;6(2):93‐8. - PubMed
McClure 2013 {published data only}
McClure 2016 {published data only}
Okuyemi 2006 {published data only}
-
- Okuyemi KS, Thomas JL, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: a pilot clinical trial. Nicotine & Tobacco Research 2006;8(5):689‐99. - PubMed
Okuyemi 2013 {published data only}
Raupach 2010 {published data only}
-
- Raupach T, Shahab L, Eimer S, Puls M, Hasenfuss G, Andreas S. Increasing the use of nicotine replacement therapy by a simple intervention: an exploratory trial. Substance Use & Misuse 2010;45(3):403‐13. - PubMed
Rigotti 2013 {published data only}
-
- Rigotti NA, Japuntich S, Regan S, Kelley JH, Chang Y, Reyen M, et al. Promoting smoking cessation after hospital discharge: the helping hand randomized controlled comparative effectiveness trial. Journal of General Internal Medicine 2013;28:S160.
Shaughnessy 1987 {published data only}
-
- Shaughnessy AF, Davis RE, Reeder CE. Nicotine chewing gum: effectiveness and the influence of patient education in a family practice. Journal of Family Practice 1987;25(3):266‐9. - PubMed
Shiffman 2000 {published data only}
-
- Shiffman S, Paty JA, Rohay JM, Marino ME, Gitchell J. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine polacrilex gum therapy. Archives of Internal Medicine 2000;160:1675‐81. - PubMed
Strecher 2005 {published data only}
-
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web‐based computer‐tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100(5):682‐8. - PubMed
Swan 2010 {published data only}
Tseng 2017 {published data only}
-
- NCT01898195. Improving adherence to smoking cessation medication among PLWHA (HIV). clinicaltrials.gov/ct2/show/NCT01898195 (first received 12 July 2013).
-
- Tseng TY, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, et al. Combining text messaging and telephone counseling to increase varenicline adherence and smoking abstinence among cigarette smokers living with HIV: a randomized controlled study. AIDS and Behavior 2017;21(7):1964‐74. - PMC - PubMed
Tønnessen 2006 {published data only}
-
- Tønnessen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334‐42. - PubMed
Willemsen 2006 {published data only}
-
- Willemsen MC, Wiebing M, Emst A, Zeeman G. Helping smokers to decide on the use of efficacious smoking cessation methods: a randomized controlled trial of a decision aid. Addiction 2006;101(3):441‐9. - PubMed
References to studies awaiting assessment
Applegate 2007 {published data only}
-
- Applegate BW, Raymond C, Collado‐Rodriguez A, Riley WT, Schneider NG. Improving adherence to nicotine gum by SMS text messaging: a pilot study. Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 Feb 21‐24; Austin (TX). 2007.
Yuhongxia 2011 {published data only}
-
- Yuhongxia L. The compliance of varenicline usage and the smoking abstinence rate via mobile phone text messaging combine with varenicline: a single‐blind, randomised control trial. Respirology 2011;16:46‐7.
References to ongoing studies
NCT02635919 {published data only}
-
- NCT02635919. Stage Ib Trial of mSMART for Smoking Cessation Medication Adherence (mSMART‐Ib). clinicaltrials.gov/ct2/show/NCT02635919 (first received 21 December 2015).
Additional references
Burns 2008
-
- Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking‐cessation attempters. American Journal of Preventive Medicine 2008;34:212‐5. - PubMed
Cahill 2016
Cheong 2010
-
- Cheong YS, Ahn SH. Effect of multi‐modal interventions for smoking cessation in a university setting: a short course of varenicline, financial incentives, e‐mail and short message service. Korean Journal of Family Medicine 2010;31:355‐60.
Coleman 2012
-
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine‐replacement therapy patches in pregnancy. New England Journal of Medicine 2012;366(9):808‐18. - PubMed
Crockett 2018
Fidler 2011
Fish 2009
GBD 2018
-
- GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923–94. - PMC - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Hajek 1999
-
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 1999;159:2033‐8. - PubMed
Hammond 2004
-
- Hammond D, McDonald PW, Fong GT, Borland R. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction 2004;99:1042‐8. - PubMed
Hartmann‐Boyce 2018
Hartmann‐Boyce 2019
Haynes 2008
Hays 2010
-
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010;12(6):574‐81. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.10 (updated March 2011). Chichester (UK): John Wiley & Sons, 2011.
Hollands 2012
Hollands 2013
Hollands 2015a
Hollands 2017
-
- Hollands GJ, Bignardi G, Johnston M, Kelly MP, Ogilvie D, Petticrew M, et al. The TIPPME intervention typology for changing environments to change behaviour. Nature Human Behaviour 2017;1:0140.
Horne 2013
Hughes 2014
Lancaster 2017
Moher 2010
Morphett 2015
Naughton 2017
-
- Naughton F. Delivering "just‐in‐time" smoking cessation support via mobile phones: current knowledge and future directions. Nicotine & Tobacco Research 2017;19(3):379‐83. - PubMed
NICE 2009
-
- National Institute for Health and Clinical Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Clinical guideline CG76. London: National Institute for Health and Clinical Excellence 2009.
Nieuwlaat 2014
Raupach 2014
-
- Raupach T, Brown J, Herbec A, Brose L, West R. A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction 2014;109(1):35‐43. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shiffman 2007
-
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102:809‐14. - PubMed
Shiffman 2008
-
- Shiffman S, Sweeney ST, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10 week randomized, double‐blind, placebo‐controlled clinical trial simulating over‐the‐counter use in adult smokers. Clinical Therapeutics 2008;30:1852‐8. - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Sumner 2018
van den Brand 2017
Webb 2006
-
- Webb TL, Sheeran P. Does changing behavioral intentions engender behaviour change? A meta‐analysis of the experimental evidence. Psychological Bulletin 2006;132(2):249‐68. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

