Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease
- PMID: 31425621
- PMCID: PMC6699648
- DOI: 10.1002/14651858.CD010233.pub3
Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease
Abstract
Background: Crohn's disease (CD) is a chronic relapsing inflammatory condition and maintenance of remission is a major issue as many patients fail to achieve remission with medical management and require surgical interventions. Purine analogues such as azathioprine (AZA) and 6-mercaptopurine (6-MP) have been used to maintain surgically-induced remission in CD, but the effectiveness, tolerability and safety of these agents remains controversial.
Objectives: To assess the efficacy and safety of purine analogues (AZA and 6-MP) for maintenance of surgically-induced remission in CD.
Search methods: We searched PubMed, MEDLINE, Embase, CENTRAL, and the Cochrane IBD Group Specialized Register from inception to 26 July 2018 (and from inception to 31 July 2019). In addition, we searched reference lists of all included studies and relevant reviews, conference proceedings and trials registers.
Selection criteria: Randomised controlled trials (RCTs) with a duration of at least three months that enrolled adults and children with surgically-induced remission of CD and compared AZA or 6-MP to no treatment, placebo or any other active intervention were considered for inclusion.
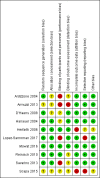
Data collection and analysis: Two authors independently assessed trial eligibility, extracted data, assessed the risk of bias and assessed the certainty of the evidence using GRADE. The primary outcome was clinical relapse. Secondary outcomes included endoscopic relapse, radiologic and surgical relapse, adverse events (AEs), serious adverse events (SAEs), withdrawal due to AEs and health-related quality of life.
Main results: Ten RCTs with a total of 928 participants were included. Study participants were adults recruited from university clinics and gastroenterology hospitals who received interventions post-surgery for a duration between 12 to 36 months. Most study participants were recruited less than three months after surgery in all except one study where participants were recruited between 6 to 24 months post-surgery. One study was rated as low risk of bias, six studies were rated high risk of bias and three were rated unclear risk of bias.There was moderate certainty evidence that purine analogues are more efficient for preventing clinical relapse than placebo. At 12 to 36 months, 51% (109/215) of AZA/6-MP participants relapsed compared to 64% (124/193) of placebo participants (RR 0.79; 95% CI 0.67 to 0.92; 408 participants; 3 studies; I² = 0%; moderate certainty evidence). The certainty of the evidence regarding the efficacy of AZA or 6-MP for maintaining postoperative clinical remission compared to 5-ASA compounds was low. At 12 to 24 months , 64% (113/177) of purine analogue participants relapsed compared to 59% (101/170) of 5-ASA participants (RR 1.05; 95% CI 0.89 to 1.24; 347 participants; 4 studies; I² = 8%; low certainty evidence). The certainty of evidence that purine analogues are inferior for preventing postsurgical clinical relapse compared to tumour necrosis factor alpha agents (anti-TNF-α) was very low. At 12 to 24 months, 43% (29/67) of AZA participants relapsed compared to 14% (10/72) of anti-TNF-α participants (RR 2.89; 95% CI 1.50 to 5.57; 139 participants; 3 studies; I² = 0%; very low certainty evidence).The effect of purine analogues compounds on AEs compared to placebo or any active treatment was uncertain, as the quality of evidence ranged from very low to low. After 12 to 24 months, 14% (12/87) of purine analogue participants experienced an AE compared to 10% (8/81) of placebo participants (RR 1.36; 95% CI 0.57 to 3.27; 168 participants; 2 studies; I² = 0%; low certainty evidence). The effect of purine analogues on AEs compared to 5-ASA agents was uncertain. After 12 to 24 months, 41% (73/176) of purine analogue participants had an AE compared to 47% (81/171) of 5-ASA participants (RR 0.89; 95% CI 0.74 to 1.07; 346 participants; 4 studies; I² = 15%; low certainty evidence). The effect of purine analogues on AEs in comparison to anti TNF-α agents was uncertain. At 12 to 24 months, 57% (32/56) of AZA participants had an AE compared to 51% (31/61) of anti-TNF-α participants (RR 1.13; 95% CI 0.83 to 1.53; 117 participants; 2 studies; I² = 0%; low certainty evidence). Purine analogue participants were more like than 5-ASA participants to have a SAE (RR 3.39, 95% CI 1.26 to 9.13, 311 participants; 3 studies; I² = 9%; very low certainty evidence), or to withdraw due to an AE (RR 2.21, 95% CI 1.28 to 3.81; 425 participants; 5 studies; I² = 0%; low certainty evidence). Commonly reported AEs across all studies included leucopenia, arthralgia, abdominal pain or severe epigastric intolerance, elevated liver enzymes, nausea and vomiting, pancreatitis, anaemia, nasopharyngitis and flatulence.
Authors' conclusions: Moderate certainty evidence suggests that AZA and 6-MP may be superior to placebo for maintenance of surgically-induced remission in participants with CD. There was no clear difference in the number of clinical relapses when purine analogues were compared with 5-ASA agents, however this is based on low certainty evidence. There was very low certainty evidence that AZA and 6-MP are more likely to result in more serious adverse events (SAEs) and withdrawals due to an AE (low certainty) when compared to 5-ASA agents. Very low certainty evidence suggests that purine analogues may be inferior to anti-TNF-α agents, however, no firm conclusions can be drawn. Further research investigating the efficacy and safety of AZA and 6-MP in comparison to other active medications in surgically-induced remission of CD is warranted.
Conflict of interest statement
Teuta Gjuladin‐Hellon: None known.
Zipporah Iheozor‐Ejiofor: None known.
Morris Gordon: Received travel fees to attend international scientific and training meeting such as DDW, Advances in IBD, ESPGHAN, BSPGHAN and Cochrane focused international events from companies including: Abbott, Nutricia, Biogaia, Ferring, Allergan, and Tillots.
Anthony K Akobeng: None known.
Figures



































Update of
-
Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2014 Aug 1;2014(8):CD010233. doi: 10.1002/14651858.CD010233.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Aug 06;8:CD010233. doi: 10.1002/14651858.CD010233.pub3. PMID: 25081347 Free PMC article. Updated.
References
References to studies included in this review
Ardizzone 2004 {published data only}
-
- Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127(3):730‐40. - PubMed
Armuzzi 2013 {published data only}
-
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Infliximab is more effective than azathioprine in the long‐term prevention of postoperative recurrence of Crohn's disease. Gastroenterology April 2015;1:S856.
-
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: A pilot study. Digestive and Liver Disease 2012;44:S194.
-
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: An open‐label pilot study. Gastroenterology 2012;142(5 Suppl 1):S780. - PubMed
-
- Armuzzi A, Felice C, Papa A, Marzo M, Pugliese D, Andrisani G, et al. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn's disease: an open‐label pilot study. Journal of Crohn's and Colitis 2013;7(12):e623‐9. - PubMed
D'Haens 2008 {published data only}
-
- D'Haens G, Vermeire S, Assche G, Noman M, Aerden I, Olmen G, et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn's disease: a controlled randomized trial. Gastroeneterology 2008;135(4):1123‐9. - PubMed
-
- D'Haens GR, Noman M, Assche GA, Olmen G, Aerden I, Vermeire S, et al. Combination therapy with metronidazole and azathioprine reduces severe postoperative recurrence of Crohn's disease: A double‐blind controlled randomized trial. Gastroenterology 2007 2007;132(4 Suppl 1):A52. - PubMed
Hanauer 2004 {published data only}
-
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. - PubMed
-
- Korelitz B, Hanauer S, Rutgeerts P, Present D, Peppercorn M. Post‐operative prophylaxis with 6‐MP, 5‐ASA or placebo inCrohn's disease: A 2 year multicenter trial. Gastroenterology 1998;114(4 Part 2):A486.
Herfarth 2006 {published data only}
-
- Dilger K, Schaeffeler E, Lukas M, Strauch U, Herfarth H, Muller R, et al. Monitoring of thiopurine methyltransferase activity in postsurgical patients with Crohn's disease during 1 year of treatment with azathioprine or mesalazine. Therapeutic Drug Monitoring 2007;29(1):1‐5. - PubMed
-
- Herfarth H, Obermeier F, Tjaden C, Lukas M, Serclova Z, Dignass AU, et al. Double‐blind, double dummy, randomized, multicentre, comparative study on the efficacy and safety of azathioprine (AZA) versus mesalazine (5‐ASA) for prevention of postoperative endoscopic recurrence in Crohn’s disease. Gastroenterology 2006;130(4 Suppl 2):A480‐1.
-
- Muller R, Herfarth H. More information on 2006 study published in Gut. Email to Morris Gordon 2/5/2012.
Lopez‐Sanroman 2017 {published data only}
-
- Lopez‐Sanroman A, Vera‐Mendoza I, Domenech E, Taxonera C, Vega V, Marin‐Jimenez I, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn’s disease recurrence. A GETECCU randomised trial. Journal of Crohn's and Colitis 2017;11(11):1293–301. - PubMed
-
- Lopez‐Sanroman A, Vera‐Mendoza I, Domenech E, Taxonera C, Vega V, Marin‐Jimenez I, et al. Apprecia: Adalimumab vs azathioprine in the prevention of Crohn's disease recurrence after surgical resection. A geteccu multicenter randomized trial. United European Gastroenterology Journal 2015;1:A3.
-
- NCT01564823. Adalimumab on Preventing Post‐chirurgic Recurrence on Crohn's Disease (APPRECIA). clinicaltrials.gov/ct2/show/NCT01564823 (accessed 28 March 2012).
-
- Taxonera C, López‐Sanromán A, Vera‐Mendoza I, Domènech E, Ruiz VV, Marín‐Jiménez I, et al. Quality of life during one year of postoperative prophylactic drug therapy after intestinal resection in Crohn's patients: Results of the APPRECIA trial. Digestive and Liver Disease 2019;51(4):529‐35. - PubMed
Mowat 2016 {published data only}
-
- Arnott I, Mowat C, Ennis H, Keerie C, Lewis S, Kennedy N, et al. The TOPPIC trial: A randomised, double‐blind parallel‐group trial of mercaptopurine versus placebo to prevent recurrence of Crohn's disease following surgical resection in 240 patients. Journal of Crohn's and Colitis 2016;10(1 Supplement):S21‐S22. - PubMed
-
- Arnott I, Mowat C, Ennis H, Keerie C, Lewis S, Kennedy N, et al. The toppic trial: A randomized, double‐blind parallel group trial of mercaptopurine vs placebo to prevent recurrence of Crohn's disease following surgical resection in 240 patients. Gastroenterology 2016;150(4 Suppl 1):S182. - PMC - PubMed
-
- ISRCTN89489788. Randomised controlled trial of 6‐Mercaptopurine (6MP) versus placebo to prevent recurrence of Crohn's disease following surgical resection. isrctn.com/ISRCTN89489788 (accessed 22 February 2007).
Reinisch 2010 {published data only}
-
- Angelberger S, Schaeffeler E, Teml A, Petritsch W, Shonova O, Lukas M, et al. Mucosal improvement in patients with moderate to severe postoperative endoscopic recurrence of Crohn's disease and azathioprine metabolite levels. Inflammatory Bowel Diseases 2013;19:590‐8. - PubMed
-
- Angelberger S, Schaeffeler E, Teml A, Petritsch W, Shonova O, Lukas M, et al. Relationship between thiopurine metabolite levels and endoscopic improvement in patients with postoperative moderate to severe endoscopic recurrence of Crohn'sdisease. Gastroenterology 2010;138(5 Suppl 1):S685. - PubMed
-
- NCT00946946. Preventing postoperative relapse in Crohn's disease patients at risk: Azathioprine versus mesalazine. clinicaltrials.gov/ct2/show/NCT00946946 (accessed 27 July 2009).
-
- Reinisch W, Angelberger S, Petritsch W, Herrlinger K, Shonova O, Lukas M, et al. A double‐blind, double‐dummy,randomized, controlled, multicenter trial on the efficacy and safety of azathioprine vs mesalamine for prevention of clinical relapses in Crohn's disease patients with postoperative moderate or severe endoscopic recurrence. Gastroenterology 2008;134(4 Suppl 1):A70.
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for the prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomized, double‐blind, double‐dummy, multicentre trial. Gut 2010;59(6):752‐9. - PubMed
Savarino 2013 {published data only}
-
- Bodini G, Pellegatta G, Giannini EG, Savarino V, Savarino EV. Adalimumab therapy rather than azathioprine and mesalamine is able to halt Crohn's disease progression after resective surgery and a post‐hoc analysis of a prospective randomized study. Gastroenterology. 2017; Vol. 152, issue 5 Supplement:S774.
-
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease ‐ a randomized trial. Digestive and Liver Disease 2013;45:S94‐5. - PubMed
-
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. American Journal of Gastroenterology 2013;108(11):1731‐42. - PubMed
-
- Savarino E, Bodini G, Dulbecco P, Marabotto E, Assandri L, Bruzzone L. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease ‐ a randomized trial. Gastroenterology 2013;144(5 Suppl 1):S21. - PubMed
Scapa 2015 {published data only}
-
- NCT01629628. Adalimumab for the Management of Post‐operative Crohn's Disease (CD) POPART. clinicaltrials.gov/ct2/show/NCT01629628 (accessed 27 June 2012).
-
- Scapa E, Maharshak N, Kariv Y, Ben‐Horin S, White ID, Santo E, et al. Early initiation of adalimumab significantly diminishes post‐operative Crohn's disease recurrence, and is superior to immunomodulator therapy. Preliminary results from the POPART trial. Gastroenterology 2015;148(4 Supplement):S240‐1.
References to studies excluded from this review
Ferrante 2015 {published data only}
-
- Ferrante M, Papamichael K, Duricova D, D'Haens G, Vermeire S, Archavlis E, et al. Systematic versus Endoscopy‐driven Treatment with Azathioprine to Prevent Postoperative Ileal Crohn's Disease Recurrence. Journal of Crohn's and Colitis 2015;9(8):617‐24. - PubMed
-
- Ferrante M, Papamichael K, Duricova D, D'Haens G, Vermeire S, Archavlis E, et al. Systematic versus endoscopy‐driven treatment with azathioprine to prevent postoperative ileal Crohn's disease recurrence. Journal of Crohn's and Colitis 2014;8:S205‐6. - PubMed
-
- Ferrante M, Papamichael K, Duricova D, D'Haens GR, Vermeire S, Archavlis EJ, et al. Systematic versus endoscopy‐driven treatment with azathioprine to prevent postoperative ileal crohn's disease recurrence: Interim results from a randomized, multicenter trial. Gastroenterology 2014;146(5 Suppl 1):S592. - PubMed
Mañosa 2013 {published data only}
-
- Manosa M, Cabre E, Bernal I, Esteve M, Garcia‐ Planella E, Ricart Gomez E. Azathioprine versus azathioprine plus metronidazole for the prevention of postoperative endoscopic recurrence of Crohn's disease: A randomized, placebo controlled trial. Journal of Crohn's and Colitis 2012;6:S93. - PubMed
-
- Mañosa M, Cabré E, Bernal I, Esteve M, Garcia‐Planella E, Ricart E, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Inflammatory Bowel Diseases 2013;19(9):1889‐95. - PubMed
NCT01876264 {published data only}
-
- NCT01876264. Crohn's extent of resection trial. clinicaltrials.gov/ct2/show/NCT01876264 (accessed 12 June 2013).
NCT02247258 {published data only}
-
- NCT02247258. Azathioprine in the prevention of ileal Crohn's disease postoperative recurrence. clinicaltrials.gov/ct2/show /NCT02247258 (accessed 23 September 2014).
Nos 2000 {published data only}
-
- Nos P, Hinojosa J, Aguilera V, Moles JR, Pastor M, Ponce J, et al. Azathiprine and 5‐ASA in the prevention of postoperative recurrence in Crohn's disease. Gastroenterología y Hepatología 2000;23(8):374‐8. - PubMed
Reinisch 2013 {published data only}
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Greinwald R, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in Crohn's disease patients with endoscopic recurrence: Follow‐up data of a randomised, double‐blind, double‐dummy, 1‐year, multicentre trial. Journal of Crohn's and Colitis 2013;7:S254. - PubMed
Robb 2015 {published data only}
-
- Robb PM, Sorrentino D. Long‐term prevention of postoperative Crohn's disease recurrence with azathioprine: the wolf in the sheep clothing. International Journal of Colorectal Disease 2015;30(2):283‐4. - PubMed
Vidigal 2014 {published data only}
Wright 2014 {published data only}
-
- Wright EK, Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany S, et al. Intestinal resection in Crohn's disease is associated with significant and durable improvement in health related quality of life although to a lesser extent in women and smokers. Results from the POCER study. Gastroenterology 2014;146(5 Suppl 1):S435.
Wright 2015 {published data only}
-
- Wright EK, Kamm MA, Cruz P, Hamilton AL, Ritchie K, Bell SJ, et al. Structured post‐operative treatment and monitoring to prevent Crohn's disease recurrence is cost effective. Results from the POCER study. Journal of gastroenterology and hepatology 2015;30:145.
Zhu 2015 {published data only}
-
- NCT01015391. Efficacy study of T2 versus AZA to maintain clinical and endoscopic remission in postoperative Crohn's disease. clinicaltrials.gov/ct2/show/NCT01015391 (accessed 18 November 2009).
-
- Zhu W, Li Y, Gong J, Zuo L, Zhang W, Cao L, et al. Tripterygium wilfordii Hook. f. versus azathioprine for prevention of postoperative recurrence in patients with Crohn's disease: a randomized clinical trial. Digestive & Liver Disease 2015;47(1):14‐9. - PubMed
References to ongoing studies
NCT03185611 {published data only}
-
- NCT03185611. Effectiveness of rifaximin combined with thiopurine on preventing postoperative recurrence in Crohn's disease. clinicaltrials.gov/ct2/show/NCT03185611 (accessed 14 June 2017).
NL1344 {published data only}
-
- NL1344. Azathioprine maintenance treatment versus infliximab maintenance treatment in Crohn’s disease patients in remission (Azorix trial). trialregister.nl/trial/1344 (accessed 1 August 2008).
Additional references
Abraham 2009
Axelrad 2016
Benchimol 2009
Bernell 2000
Boreinstein 2009
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2009.
Camus 2013
-
- Camus M, Seksik P, Bourrier A, Nion‐Larmurier I, Sokol H. Long‐term outcome of patients with Crohn’s disease who respond to azathioprine. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 2013;11(4):389‐394. - PubMed
Chande 2015
Colonna 1994
-
- Colonna T, Korelitz BI. The role of leukopenia in the 6‐mercaptopurine induced remission of refractory Crohn's disease. American Journal of Gastroenterology 1994;89(3):362‐6. - PubMed
Doherty 2009
Egger 2001
-
- Egger M, Davey‐Smith G, Altman D (Editors). Systematic reviews in health care: Meta‐analysis in context. Second Edition. London.: BMJ Publishing Group, 2001.
Fraser 2002
Gionchetti 2016
-
- Gionchetti P, Dignass A, Danese S, Dias FJM, Rogler G, Lakatos PL, et al. on behalf of ECCO. 3rd European Evidenced‐based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2; Surgical Management and Special Situations. Journal of Crohn's and Colitis February 2017;11(2):135‐49. - PubMed
Gjuladin‐Hellon 2019
Gomollon 2017
-
- Gomollón F, Dignass A, Annese V, Tilg H, Assche G, Lindsay JO, et al. 3rd European evidence‐based consensus on the management of Crohn’s disease 2016: Part 1: Diagnosis and medical management.. Journal of Crohn's & Colitis 2017;11:3‐25. - PubMed
Gordon 2011
Guyatt 2008
Hafraoui 2002
-
- Hafraoui S, Dewit O, Marteau P, Cosnes J, Colombel JF, Modigliani R, et al. Mycophenolate mofetil in refractory Crohn's disease after failure of treatments by azathioprine or methotrexate [Le mycophénolate mofétil dans les formes chroniques actives de la maladie de Crohn après échec de lazathioprine ou du méthotrexate]. Gastroenterologie Clinique et Biologique 2002;26(1):17‐22. - PubMed
Hanauer 2001
-
- Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. American Journal of Gastroenterology 2001;96(3):635‐43. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lennard 1989
-
- Lennard L, Loon JA, Weinshilboum RM. Pharmacogenitics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clinical Pharmacology and Therapeutics 1989;46(2):149‐54. - PubMed
Lennard 1992
-
- Lennard L. The clinical pharmacology of 6‐mercaptopurine. European Journal of Clinical Pharmacology 1992;43(4):329‐39. - PubMed
NICE 2016
-
- National Institute of Health and Care Excellence. Crohn's Disease: management (CG152). Available: https://www.nice.org.uk/guidance/cg152/evidence/addendum‐pdf‐2489565421 2016.
Regueiro 2009
-
- Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pasci M, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology 2009;136(2):441–50. - PubMed
Rolfe 2006
Rutgeerts 1990
-
- Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99(4):956‐63. - PubMed
Sahasranaman 2008
-
- Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. European Journal ofClinical Pharmacology 2008;64:753‐67. - PubMed
Sandborn 1996
-
- Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6‐mercaptopurine, cyclosporine, and methotrexate. American Journal of Gastroenterology 1996;91(3):423‐33. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Steinhart 2003
Sutton 2000
-
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta‐analysis in medical research. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2000.
Timmer 2016
Weinshilboum 1980
Williams 1990
-
- Williams JG, Wong WD, Rothenberger DA, Goldberg SM. Recurrence of Crohn's disease after resection. British Journal of Surgery 1990;78(1):10‐9. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

