Motivational interviewing for smoking cessation
- PMID: 31425622
- PMCID: PMC6699669
- DOI: 10.1002/14651858.CD006936.pub4
Motivational interviewing for smoking cessation
Abstract
Background: Motivational Interviewing (MI) is a directive patient-centred style of counselling, designed to help people to explore and resolve ambivalence about behaviour change. It was developed as a treatment for alcohol abuse, but may help people to a make a successful attempt to stop smoking.
Objectives: To evaluate the efficacy of MI for smoking cessation compared with no treatment, in addition to another form of smoking cessation treatment, and compared with other types of smoking cessation treatment. We also investigated whether more intensive MI is more effective than less intensive MI for smoking cessation.
Search methods: We searched the Cochrane Tobacco Addiction Group Specialised Register for studies using the term motivat* NEAR2 (interview* OR enhanc* OR session* OR counsel* OR practi* OR behav*) in the title or abstract, or motivation* as a keyword. We also searched trial registries to identify unpublished studies. Date of the most recent search: August 2018.
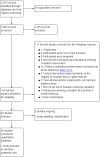
Selection criteria: Randomised controlled trials in which MI or its variants were offered to smokers to assist smoking cessation. We excluded trials that did not assess cessation as an outcome, with follow-up less than six months, and with additional non-MI intervention components not matched between arms. We excluded trials in pregnant women as these are covered elsewhere.
Data collection and analysis: We followed standard Cochrane methods. Smoking cessation was measured after at least six months, using the most rigorous definition available, on an intention-to-treat basis. We calculated risk ratios (RR) and 95% confidence intervals (CI) for smoking cessation for each study, where possible. We grouped eligible studies according to the type of comparison. We carried out meta-analyses where appropriate, using Mantel-Haenszel random-effects models. We extracted data on mental health outcomes and quality of life and summarised these narratively.
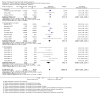
Main results: We identified 37 eligible studies involving over 15,000 participants who smoked tobacco. The majority of studies recruited participants with particular characteristics, often from groups of people who are less likely to seek support to stop smoking than the general population. Although a few studies recruited participants who intended to stop smoking soon or had no intentions to quit, most recruited a population without regard to their intention to quit. MI was conducted in one to 12 sessions, with the total duration of MI ranging from five to 315 minutes across studies. We judged four of the 37 studies to be at low risk of bias, and 11 to be at high risk, but restricting the analysis only to those studies at low or unclear risk did not significantly alter results, apart from in one case - our analysis comparing higher to lower intensity MI.We found low-certainty evidence, limited by risk of bias and imprecision, comparing the effect of MI to no treatment for smoking cessation (RR = 0.84, 95% CI 0.63 to 1.12; I2 = 0%; adjusted N = 684). One study was excluded from this analysis as the participants recruited (incarcerated men) were not comparable to the other participants included in the analysis, resulting in substantial statistical heterogeneity when all studies were pooled (I2 = 87%). Enhancing existing smoking cessation support with additional MI, compared with existing support alone, gave an RR of 1.07 (95% CI 0.85 to 1.36; adjusted N = 4167; I2 = 47%), and MI compared with other forms of smoking cessation support gave an RR of 1.24 (95% CI 0.91 to 1.69; I2 = 54%; N = 5192). We judged both of these estimates to be of low certainty due to heterogeneity and imprecision. Low-certainty evidence detected a benefit of higher intensity MI when compared with lower intensity MI (RR 1.23, 95% CI 1.11 to 1.37; adjusted N = 5620; I2 = 0%). The evidence was limited because three of the five studies in this comparison were at risk of bias. Excluding them gave an RR of 1.00 (95% CI 0.65 to 1.54; I2 = n/a; N = 482), changing the interpretation of the results.Mental health and quality of life outcomes were reported in only one study, providing little evidence on whether MI improves mental well-being.
Authors' conclusions: There is insufficient evidence to show whether or not MI helps people to stop smoking compared with no intervention, as an addition to other types of behavioural support for smoking cessation, or compared with other types of behavioural support for smoking cessation. It is also unclear whether more intensive MI is more effective than less intensive MI. All estimates of treatment effect were of low certainty because of concerns about bias in the trials, imprecision and inconsistency. Consequently, future trials are likely to change these conclusions. There is almost no evidence on whether MI for smoking cessation improves mental well-being.
Conflict of interest statement
AF is employed by the University of Oxford to work as a Senior Researcher in the Health Behaviours group on projects related to nicotine consumption. Anne has been the recipient of a grant funded by Cancer Research UK to explore clinician attitudes towards electronic cigarettes. None of this is deemed a conflict of interest.
JL has no known conflicts of interest.
NL is employed by the University of Oxford to work as Managing Editor for the Cochrane Tobacco Addiction Group (TAG). TAG's infrastructure is funded by the NIHR. Nicola has received payment for lectures on systematic review methodology, and has been an applicant on project funding to carry out priority setting and systematic reviews in the area of tobacco control (NIHR funded). None of this is deemed a conflict of interest.
PA has no known conflicts of interest.
TT has no known conflicts of interest.
Figures

















Update of
- doi: 10.1002/14651858.CD006936.pub3
References
References to studies included in this review
Ahluwalia 2006 {published data only}
-
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction 2006;101(6):883‐91. - PubMed
-
- Ahluwalia JS, Okuyemi KS, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers (PA7‐6). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 Feb 15‐18; Orlando (FL). 2006.
-
- Ahluwalia JS, Richter K, Mayo MS, Ahluwalia HK, Choi WS, Schmelzle KH, et al. African American smokers interested and eligible for a smoking cessation clinical trial: predictors of not returning for randomization. Annals of Epidemiology 2002;12(3):206‐12. - PubMed
-
- Nollen N, Ahluwalia JS, Okuyemi K, Choi W, Kaur H, Mayo M. Kick it at SWOPE II (KIS II): baseline data from a smoking cessation trial among African American (AA) light smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20‐23; Prague, Czech Republic. 2005.
Audrain‐McGovern 2011 {published data only}
-
- Audrain‐McGovern J, Stevens S, Murray PJ, Kinsman S, Zuckoff A, Pletcher J, et al. The efficacy of motivational interviewing versus brief advice for adolescent smoking behavior change. Pediatrics 2011;128(1):e101‐11. - PubMed
-
- Kalkhuis‐Beam S, Stevens SL, Baumritter A, Carlson EC, Pletcher JR, Rodriguez D, et al. Participant‐ and study‐related characteristics predicting treatment completion and study retention in an adolescent smoking cessation trial. Journal of Adolescent Health 2011;49(4):371‐8. - PubMed
-
- NCT00381329. Pennsylvania Adolescent Smoking Study (PASStudy). ClinicalTrials.gov/show/NCT00381329 (first received 27 September 2006).
Bastian 2013 {published data only}
-
- Bastian LA, Fish LJ, Peterson BL, Biddle AK, Garst J, Lyna P, et al. Assessment of the impact of adjunctive proactive telephone counseling to promote smoking cessation among lung cancer patients' social networks. American Journal of Health Promotion 2013;27(3):181‐90. [] - PubMed
Battaglia 2016 {published and unpublished data}
-
- Battaglia C, Peterson J, Whitfield E, Min S‐J, Benson SL, Maddox TM, et al. Integrating motivational interviewing into a home telehealth program for veterans with posttraumatic stress disorder who smoke: a randomized controlled trial. Journal of Clinical Psychology 2016;72(3):194‐206. - PubMed
Bock 2008 {published data only}
-
- Becker BM, Bock BC, Partridge R. Smoking cessation interventions in the emergency department for smokers with chest pain. Academic Emergency Medicine 2003;10:507.
-
- Bock BC, Becker BM, Niaura RS, Partridge R, Fava JL, Trask P. Smoking cessation among patients in an emergency chest pain observation unit: outcomes of the Chest Pain Smoking Study (CPSS). Nicotine & Tobacco Research 2008;10(10):1523‐31. - PubMed
Bock 2014 {published and unpublished data}
-
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low‐income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413‐22. [CENTRAL: 986341; CRS: 9400050000000050; EMBASE: 2014185069; PUBMED: 24174612] - PMC - PubMed
Butler 1999 {published data only}
-
- Butler CC, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. British Journal of General Practice 1999;49(445):611–6.
Catley 2016 {published data only}
-
- NCT01188018. Testing counseling styles to motivate smokers to quit. ClinicalTrials.gov/show/NCT01188018 (first received 25 August 2010).
Colby 2005 {published data only}
-
- Colby SM, Monti PM, O'Leary‐Tevyaw T, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in medical settings. Addictive Behaviors 2005;30(5):865‐74. - PubMed
Colby 2012 {published data only}
Cook 2016 {published data only}
-
- NCT01122238. Identifying treatments to motivate smokers to quit. ClinicalTrials.gov/show/NCT01122238 (first received 17 March 2015).
Davis 2011 {published data only}
-
- Davis MF, Shapiro D, Windsor R, Whalen P, Rhode R, Miller HS, et al. Motivational interviewing versus prescriptive advice for smokers who are not ready to quit. Patient Education and Counseling 2011;83(1):129‐33. - PubMed
De Azevedo 2010 {published data only}
-
- Azevedo RC, Mauro ML, Lima DD, Gaspar KC, Silva VF, Botega NJ. General hospital admission as an opportunity for smoking‐cessation strategies: a clinical trial in Brazil. General Hospital Psychiatry 2010;32(6):599‐606. - PubMed
Demétrio Faustino‐Silva 2018 {published data only}
-
- Demétrio Faustino‐Silva D, Melnick R, Keller Celeste R. Efficacy of motivational interviewing in smoking groups in primary health care: a randomized clinical trial. International Journal of Behavioral Medicine 2018;25(Suppl 1):S68.
-
- NCT03221010. Use of the Motivational Interviewing in the Treatment of smokers in Groups in primary health care (MITG). clinicaltrials.gov/ct2/show/NCT03221010 (first received 18 July 2017).
Ellerbeck 2009 {published data only}
Harris 2010 {published data only}
Helstrom 2007 {published data only}
Hollis 2007 {published data only}
Kelly 2006 {published data only}
-
- Kelly AB, Lapworth K. The HYP program‐targeted motivational interviewing for adolescent violations of school tobacco policy. Preventive Medicine 2006;43(6):466‐71. - PubMed
Lewis 1998 {published data only}
-
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: a randomized clinical trial. Preventive Medicine 1998;27(2):296‐303. - PubMed
Lloyd‐Richardson 2009 {published data only (unpublished sought but not used)}
-
- Niaura R, Richardson EE, Stanton C, Carton‐Lopez S, Morrow K, Shadel W. Motivation and patch treatment for HIV‐positive smokers: psychosocial barriers to cessation (POS1‐057). Society for Research on Nicotine and Tobacco 10th Annual Meeting; 2004 Feb 18‐21; Phoenix, Arizona. 2004.
Louwagie 2014 {published data only}
-
- Louwagie GM, Okuyemi KS, Olalekan AA. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction 2014;109(11):1942‐52. - PubMed
-
- Louwagie GMC, Ayo‐Yusuf OA. Predictors of tobacco smoking abstinence among tuberculosis patients in South Africa CNO ‐ CN‐01165925. Journal of Behavioral Medicine 2015;38(3):472‐82. - PubMed
Marshall 2016 {published data only (unpublished sought but not used)}
-
- Marshall HM, Courtney DA, Passmore LH, McCaul EM, Yang IA, Bowman RV, et al. Brief tailored smoking cessation counseling in a lung cancer screening population is feasible: a pilot randomized controlled trial.. Nicotine & Tobacco Research 2016;18(7):1665‐9. [DOI: ] - PubMed
-
- Marshall HM, Yang IA, Passmore L, McCaul EM, Bowman R, Fong KM. A randomized controlled trial of brief counselling intervention and audio materials for smoking cessation in a low‐dose CT screening study. Journal of Thoracic Oncology 2013;8(Suppl 2):S703.
Matuszewski 2018 {published data only}
-
- Matuszewski P, Ordonio K, O’Hara N, O’Toole R. Prospective randomized trial on smoking cessation in orthopaedic trauma patients: could the 'Hawthorne Effect' of carbon monoxide monitoring be the best treatment?. ota.org/education/meetings‐and‐courses/abstracts/prospective‐randomized‐... (Accessed 11 May 2019).
-
- NCT02428244. Let's STOP now trial: smoking in trauma orthopaedic patients. ClinicalTrials.gov/show/NCT02428244 (first received 4 July 2011).
McClure 2005 {published data only}
-
- McClure J, Westbrook E, Wetter DW, Curry SJ. Proactive cessation treatment in a managed care setting. Society for Behavioral Medicine 10th Annual Meeting; 2005 Feb 18‐21; Phoenix, Arizona. 2005.
-
- McClure J, Wetter D, Curry SJ. Acceptability of proactive contact and counseling among female smokers (POS3‐039). Nicotine & Tobacco Research 2004;6:722.
-
- McClure JB, Westbrook E, Curry SJ, Wetter DW. Proactive, motivationally enhanced smoking cessation counseling among women with elevated cervical cancer risk. Nicotine & Tobacco Research 2005;7(6):881‐9. - PubMed
Naik 2014 {published and unpublished data}
-
- Naik S, Khanagar S, Kumar A, Ramachandra S, Vadavadagi SV, Dhananjaya KM. Assessment of effectiveness of smoking cessation intervention among male prisoners in India: a randomized controlled trial. Journal of International Society of Preventive & Community Dentistry 2014;4(Suppl 2):S110‐5. - PMC - PubMed
NCT02645838 {published data only}
-
- NCT02645838. Motivational interviewing for smoking cessation. clinicaltrials.gov/ct2/show/NCT02645838 (first received 5 January 2016).
Okuyemi 2013 {published data only}
-
- NCT00786149. Improving varenicline adherence and outcomes in homeless smokers. ClinicalTrials.gov/show/NCT00786149 (first received 6 November 2008).
Rohsenow 2014 {published data only}
-
- Rohsenow DJ, Monti PM, Colby SM, Martin RA. Brief interventions for smoking cessation in alcoholic smokers. Alcoholism, Clinical and Experimental Research 2002;26(12):1950‐1. - PubMed
Rohsenow 2015 {published data only}
-
- Rohsenow D, Martin R, Tidey J, Monti P, Swift R. Motivational and contingency interventions for unmotivated smokers in substance abuse treatment (PA‐7). Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 Mar 20‐23; Prague, Czech Republic. 2005.
Sherman 2016 {published data only}
-
- NCT01363245. Effectiveness of smoking‐cessation interventions for urban hospital patients. clinicaltrials.gov/ct2/show/NCT01363245 (first received 1 June 2011).
Soria 2006 {published data only}
Stein 2006 {published data only}
-
- Stein MD, Anderson BJ, Niaura R. Smoking cessation patterns in methadone‐maintained smokers. Nicotine & Tobacco Research 2007;9(3):421‐8. - PubMed
-
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone‐maintained. Addiction 2006;101(4):599‐607. - PubMed
Tevyaw 2009 {published data only}
Vidrine 2019 {published data only}
-
- NCT00948129. Smoking cessation for low‐income adults. clinicaltrials.gov/ct2/show/NCT00948129 (first received 29 July 2009).
-
- Vidrine DJ, Frank‐Pearce SG, Vidrine JI, Tahay PD, Marani SK, Chen S, et al. Efficacy of mobile phone–delivered smoking cessation interventions for socioeconomically disadvantaged individuals: a randomized clinical trial. JAMA Internal Medicine 2019;179(2):167‐74. [DOI: 10.1001/jamainternmed.2018.5713] - DOI - PMC - PubMed
Woodruff 2007 {published data only}
-
- Woodruff SI, Conway TL, Edwards CC, Elliott SP, Crittenden J. Evaluation of an Internet virtual world chat room for adolescent smoking cessation. Addictive Behaviors 2007;32(9):1769‐86. - PubMed
References to studies excluded from this review
ACTRN12609000627257 {published data only}
-
- ACTRN12609000627257. Using admissions to a "smokefree" hospital to promote cessation of smoking in mental health inpatients versus a representation of the general population [The effect of a quit smoking program on the intention of mental health inpatients versus orthopaedic, plastic surgery and neurosurgery inpatients (as representative of the general population) to adopt smoking cessation strategies]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308251 (first received 20 Jul 2009).
ACTRN12609001039279 {published data only}
-
- ACTRN12609001039279. Healthy lifestyle intervention for cardiovascular disease risk reduction among smokers with psychotic disorders [In smokers with psychotic disorders, is an intervention consisting of nicotine replacement therapy (NRT) and motivational interviewing and cognitive‐behaviour therapy (MICBT) and contingency management (CM) as or more effective as a one‐session intervention followed by NRT plus brief contact for reduction of cardiovascular disease (CVD) risk and smoking cessation?]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609001039279 (first received 17 Jul 2009).
ACTRN12612000016831 {published data only}
-
- ACTRN12612000016831. A secondary prevention patient centred model for smoking cessation [A pilot study of a nurse‐led evidence based smoking cessation program in smokers with cardiovascular disease to develop a secondary prevention model of care for smoking cessation]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000016831 (first received 21 Dec 2011).
ACTRN12614000876695 {published data only}
-
- ACTRN12614000876695. Improving radiotherapy outcomes with smoking cessation: feasibility trial in head and neck cancer patients [Improving radiotherapy outcomes in head and neck cancer patients: a preliminary comparison of smoking cessation intervention ‘Varenicline plus support’ with ‘treatment as usual’]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366605 (first received 7 Jul 2014).
ACTRN12614001147673 {published data only}
-
- ACTRN12614001147673. Smoke‐free recovery from trauma surgery: a pilot trial of an online smoking cessation programme for trauma surgery patients [A pilot trial of an online smoking cessation program investigating uptake and usage of an online smoking cessation programme]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366829 (first received 14 Oct 2014).
ACTRN12616000314426 {published data only}
-
- ACTRN12616000314426. Efficacy of a pre‐release group‐based abstinence program on extending prisoners' smoking abstinence post release from smoke‐free prisons in Queensland [Efficacy of a pre‐release group‐based abstinence program on extending prisoners' smoking abstinence post release from smoke‐free prisons in Queensland: a randomised controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370213 (first received 25 Feb 2016).
Aertsen Van Der Kuip 2006 {published data only}
-
- Aertsen‐Van Der Kuip MSA, Besselink RM, Vlugt MJ, Peters E, Fouwels AJ, Wollersheim H, et al. Successful motivational interviewing for smoking cessation in a secondary cardiovascular prevention clinic. European Heart Journal 2006;27(Suppl 1):165.
Ahluwalia 1998 {published data only}
-
- Ahluwalia JS, Resnicow K, Clark WS. Knowledge about smoking, reasons for smoking, and reasons for wishing to quit in inner‐city African Americans. Ethnicity & Disease 1998;8(3):385‐93. - PubMed
Auer 2016 {published data only}
-
- Auer R, Gencer B, Tango R, Nanchen D, Matter CM, Luscher TF, et al. Uptake and efficacy of a systematic intensive smoking cessation intervention using motivational interviewing for smokers hospitalised for an acute coronary syndrome: a multicentre before‐after study with parallel group comparisons. BMJ Open 2016;6(9):e011520. [DOI: 10.1136/bmjopen-2016-011520] - DOI - PMC - PubMed
Baker 2006 {published data only}
-
- Baker A, Richmond R, Kay‐Lambkin F, Lewin TJ, Carr VJ. Randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder: 3‐year follow‐up (PA2‐2). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007:19.
-
- Lan TH, Chiu HJ, Wu BJ, Hung TH, Hu TM. Readiness to quit and smoking reduction outcomes. American Journal of Psychiatry 2007;164:827‐8. - PubMed
Bernstein 2018 {published data only}
Boccio 2017 {published data only}
Bolger 2010 {published data only}
-
- Bolger K, Carter K, Curtin L, Martz DM, Gagnon SG, Michael KD. Motivational interviewing for smoking cessation among college students. Journal of College Student Psychotherapy 2010;24:116‐29.
Bonevski 2018 {published data only}
-
- Bonevski B, Twyman L, Paul C, D'Este C, West R, Siahpush M, et al. Smoking cessation intervention delivered by social service organisations for a diverse population of Australian disadvantaged smokers: a pragmatic randomised controlled trial. Preventive Medicine 2018;112:38‐44. - PubMed
Borrelli 2002 {published data only}
-
- Borrelli B, McQuaid EL, Becker B, Hammond K, Papandonatos G, Fritz G, et al. Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Education Research 2002;17(5):659‐69. - PubMed
Borrelli 2005 {published data only}
-
- Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community‐nurse Assisted Research and Education on Smoking). Preventive Medicine 2005;41(5‐6):815‐21. [DOI: 10.1016/j.ypmed.2005.08.004] - DOI - PubMed
Borrelli 2016 {published data only}
-
- Borrelli B, McQuaid EL, Tooley EM, Busch AM, Hammond SK, Becker B, et al. Motivating parents of kids with asthma to quit smoking: the effect of the teachable moment and increasing intervention intensity using a longitudinal randomized trial design. Addiction 2016;111(9):1646‐55. [DOI: 10.1111/add.13389] - DOI - PMC - PubMed
Borrelli 2017 {published data only}
-
- Borrelli B, Endrighi R, Hammond SK, Dunsiger S. Smokers who are unmotivated to quit and have a child with asthma are more likely to quit with intensive motivational interviewing and repeated biomarker feedback CNO ‐ CN‐01430706. Journal of Consulting and Clinical Psychology 2017;85(11):1019‐28. [DOI: 10.1037/ccp0000238] - DOI - PMC - PubMed
Boyle 2007 {published data only}
Breland 2014 {published data only}
-
- Breland AB, Almond L, Kienzle J, Ondersma SJ, Hart AJr, Weaver M, et al. Targeting tobacco in a community‐based addiction recovery cohort: results from a computerized, brief, randomized intervention trial. Contemporary Clinical Trials 2014;38(1):113‐20. [DOI: 10.1016/j.cct.2014.03.008] - DOI - PubMed
Bronson 1989 {published data only}
-
- Bronson DL, Flynn BS, Solomon LJ, Vacek PM, Secker‐Walker RH. Smoking cessation counseling during periodic health examinations. Archives of Internal Medicine 1989;149(7):1653‐6. - PubMed
Brooks 2017 {published data only}
Brown 2003 {published data only}
-
- Brown RA, Strong DR, Abrantes AM, Myers MG, Ramsey SE, Kahler CW. Effects on substance use outcomes in adolescents receiving motivational interviewing for smoking cessation during psychiatric hospitalization. Addictive Behaviors 2009;34(10):887‐91. [DOI: 10.1016/j.addbeh.2009.03.003] - DOI - PMC - PubMed
-
- Macpherson L, Strong DR, Palm KM, Abrantes AM, Brown RA. Post‐intervention lapse and relapse in adolescent smokers with psychiatric comorbidity (POS1‐84). Society for Research on Nicotine and Tobacco 13th Annual Meeting, 2007 February 21‐24, Austin, Texas. 2007:58.
Caponnetto 2017 {published data only}
Carpenter 2004 {published data only}
-
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology 2004;72(3):371‐81. [DOI: 10.1037/0022-006X.72.3.371] - DOI - PubMed
Cigrang 2002 {published data only}
Collicott 2001 {published data only}
-
- Collicott SF. Increasing readiness to change among smokers in a primary care setting. Dissertation Abstracts International 2001;61:6699.
Cornuz 2002 {published data only}
-
- Cornuz J, Humair JP, Seematter L, Stoianov R, Melle G, Stalder H, et al. Efficacy of resident training in smoking cessation: a randomized, controlled trial of a program based on application of behavioral theory and practice with standardized patients. Annals of Internal Medicine 2002;136(6):429‐37. - PubMed
Curry 2003 {published data only}
-
- Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric‐based smoking cessation intervention for low‐income women: a randomized trial. Archives of Pediatrics & Adolescent Medicine 2003;157(3):295‐302. - PubMed
Dornelas 2000 {published data only}
-
- Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Preventive Medicine 2000;30(4):261‐8. - PubMed
Eakin 2014 {published data only}
-
- Eakin MN, Rand CS, Borrelli B, Bilderback A, Hovell M, Riekert KA. Effectiveness of motivational interviewing to reduce head start children's secondhand smoke exposure: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1530‐7. [DOI: 10.1164/rccm.201404-0618OC] - DOI - PMC - PubMed
Emmons 2001 {published data only}
-
- Emmons KM, Hammond SK, Fava J, Velicer W, Evans J, Monroe A. The impact of motivational interviewing on passive smoke reduction in low income households with young children. 11th World Conference on Tobacco or Health; 2000 August 6‐11; Chicago. 2000:142.
-
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low‐income households with young children. Pediatrics 2001;108(1):18‐24. - PubMed
Ershoff 1999 {published data only}
-
- Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking‐cessation trial: when more isn't better, what is enough?. American Journal of Preventive Medicine 1999;17(3):161‐8. - PubMed
Gariti 2002 {published data only}
-
- Gariti P, Alterman A, Mulvaney F, Mechanic K, Dhopesh V, Yu E, et al. Nicotine intervention during detoxification and treatment for other substance use. American Journal of Drug and Alcohol Abuse 2002;28:671‐9. - PubMed
George 2000 {published data only}
Glasgow 2000 {published data only}
Ha 2012 {published data only}
Ha 2015 {published data only}
Haas 2015 {published data only}
Hennrikus 2002 {published data only}
Hennrikus 2005 {published data only}
Hokanson 2006 {published data only}
Horn 2007 {published data only}
-
- Horn K, Dino G, Hamilton C, Noerachmanto N, Zhang J. Feasibility of a smoking cessation intervention for teens in the emergency department: reach, implementation fidelity, and acceptability. American Journal of Critical Care 2008;17(3):205‐16. - PubMed
Huang 2015 {published data only}
-
- Huang FF, Jiao NN, Zhang LY, Lei Y, Zhang JP. Effects of a family‐assisted smoking cessation intervention based on motivational interviewing among low‐motivated smokers in China. Patient Education and Counseling 2015;98(8):984‐90. [DOI: ] - PubMed
Hughes 2017 {published data only}
Hutchinson 2017 {published data only}
-
- Hutchinson S, Breukelen G, Schayck O, Essers B, Muris J, Feron F, et al. A randomised trial to stop passive smoking in children with a high risk of asthma (abstract). European Respiratory Journal 2014;44:1404.
-
- Hutchinson SG, Breukelen G, Schayck CP, Essers B, Hammond SK, Muris JWM, et al. Motivational interviewing and urine cotinine feedback to stop passive smoke exposure in children predisposed to asthma: a randomised controlled trial. Scientific Reports 2017;7(1):15473. [DOI: 10.1038/s41598-017-15158-2] - DOI - PMC - PubMed
Hyman 2007 {published data only}
Idrisov 2013 {published data only}
Ingersoll 2005 {published data only}
-
- Ingersoll KS, Cropsey KL, Heckman CJ. Reducing smoking among people with HIV: outcomes and lessons from three pilot projects. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20‐23; Prague, Czech Republic. 2005.
IRCT2017080435257N1 {published data only}
-
- IRCT2017080435257N1. The effect of motivational counseling on smoking cessation. en.irct.ir/trial/26698 (first received September 11 2017).
ISRCTN11353250 {published data only}
-
- ISRCTN11353250. Efficacy of smoking cessation treatment interventions for individuals with severe mental illness: a pilot randomized controlled clinical trial. www.isrctn.com/ISRCTN11353250 (first received February 6 2015).
ISRCTN50627997 {published data only}
-
- ISRCTN50627997. Evaluation of the effectiveness of brief counseling for tobacco cessation during dental care in Sweden. www.isrctn.com/ISRCTN50627997 (first received July 23 2012).
Klemperer 2017 {published data only}
Krigel 2011 {published data only}
-
- Krigel SW. Motivational interviewing and the effect of the decisional balance exercise on college smokers. Dissertation Abstracts International 2011;71:7067.
Lasser 2012 {published data only}
-
- Lasser KE, Kenst KS, Quintiliani L, Wiener RS, Murillo J, Bowen DJ. Patient navigation to promote smoking cessation in primary care: baseline results from a pilot randomized controlled trial. Journal of General Internal Medicine 2012;27:S263.
Lennox 1998 {published data only}
-
- Lennox AS, Bain N, Taylor RJ, McKie L, Donnan PT, Groves J. Stages of change training for opportunistic smoking intervention by the primary health care team. Part I: randomised controlled trial of the effect of training on patient smoking outcomes and health professional behaviour as recalled by patients. Health Education Journal 1998;57:140‐9.
Lindqvist 2013 {published data only}
Luna 2005 {published data only}
-
- Luna L. The effectiveness of motivational enhancement therapy on smoking cessation in college students. Dissertation Abstracts International: Section B: the Sciences and Engineering 2005;65:5383.
Ma 2005a {published data only}
-
- Ma GX, Tan Y, Knauer CA, Toubbeh J, Choi J. A study of culturally appropriate smoking cessation for underserved Korean American smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 2005 March 20‐23; Prague, Czech Republic. 2005.
Ma 2005b {published data only}
-
- Ma GX. Study of culturally appropriate smoking cessation for underserved Korean American smokers. American Public Health Association 133rd Annual Meeting & Exposition; 2005 Dec 10; Philadelphia, MA. 2005.
Mahajan 2017 {published data only}
-
- Mahajan R, Solanki J, Kurdekar RS, Gupta S, Modh A, Yadav O. Educating the handicraft factory workers about tobacco cessation and to assess its effectiveness by motivational interviewing: an Intervention study. Journal of Experimental Therapeutics & Oncology 2017;12(1):43‐9. - PubMed
Manfredi 1999 {published data only}
Manfredi 2004 {published data only}
-
- Manfredi C, Crittenden KS, Cho YI, Gao S. Long‐term effects (up to 18 months) of a smoking cessation program among women smokers in public health clinics. Preventive Medicine 2004;38:10‐9. - PubMed
Martin‐Lujan 2011 {published data only}
-
- Martin‐Lujan F, Piñol‐Moreso JL, Martin‐Vergara N, Basora‐Gallisa J, Pascual‐Palacios I, Sagarra‐Alamo R, et al. Effectiveness of a structured motivational intervention including smoking cessation advice and spirometry information in the primary care setting: the ESPITAP study. BMC Public Health 2011;11(1):859‐67. - PMC - PubMed
Mazas 2007 {published data only}
-
- Mazas C, Li Y, Cofta‐Woerpel L, Wetter DW, Daza P, Nguyen L, et al. Disparities and smoking cessation among Latinos (SYM 2B). Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24, Austin, Texas. 2007:3.
McCambridge 2005 {published data only}
Menzie 2018 {published data only}
-
- Menzie NS. A motivational interviewing intervention to increase utilization of smoking cessation services among veterans undergoing substance use treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2018;79:No pagination specified.
Metse 2017 {published data only}
-
- Metse AP, Wiggers J, Wye P, Wolfenden L, Freund M, Clancy R, et al. Efficacy of a universal smoking cessation intervention initiated in inpatient psychiatry and continued post‐discharge: a randomised controlled trial. Australian and New Zealand Journal of Psychiatry 2017;51(4):366‐81. [DOI: 10.1177/0004867417692424] - DOI - PubMed
Metz 2006a {published and unpublished data}
-
- Metz K, Kroeger C, Schuetz C, Floeter S, Donath C. Which smoking cessation intervention works for smokers with an alcohol addiction?. 68th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2006. 2006.
Metz 2006b {published data only}
-
- Metz K, Kroger C, Donath C, Floter S, Gradl S, Piontek D. Evaluation of a motivational intervention for smokers in rehabilitation centers. Verhaltenstherapie & Verhaltensmedizin 2006;27:445‐63.
Meyer 2003 {published data only}
-
- Meyer C, Ulbricht S, Schumann A, Hannover W, Hapke U, Rumpf HJ, et al. Interventions fostering the motivation to quit for smokers in general practice. Suchtmedizin in Forschung und Praxis 2003;5:134‐6.
-
- Ulrich JU, Ulbricht S, Goeze C, Meyer C. Brief intervention to motivate smokers to quit in primary medical care. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18:S40.
Mujcic 2018 {published data only}
NCT00169260 {published data only}
-
- NCT00169260. Proactive health intervention for tobacco users (Get PHIT). clinicaltrials.gov/ct2/show/NCT00169260 (first received September 15 2005).
NCT00701896 {published data only}
-
- NCT00701896. Smoking cessation and the natural history of HIV‐associated emphysema. clinicaltrials.gov/show/nct00701896 (first received June 19 2008).
NCT00907309 {published data only}
-
- NCT00907309. Dental and medical office internet/intranet motivational enhancement therapy to reduce teen tobacco, alcohol, and drug use. clinicaltrials.gov/show/NCT00907309 (first received May 22 2009).
NCT01098955 {published data only}
-
- NCT01098955. Smoking cessation treatment for head & neck cancer patients: acceptance and commitment therapy. clinicaltrials.gov/show/nct01098955 (first received April 5 2010).
NCT01846910 {published data only}
-
- NCT01846910. Prepare to quit ‐ a randomized clinical trial to help people quit smoking in a community dental clinic setting. clinicaltrials.gov/show/NCT01846910 (first received May 6 2014).
NCT01982617 {published data only}
-
- NCT01982617. A randomized, controlled trial of motivational interviewing compared to psychoeducation for smoking precontemplators with severe mental illness. clinicaltrials.gov/show/nct01982617 (first received November 13 2013).
NCT02086162 {published data only}
-
- NCT02086162. Randomized controlled trial (RCT) of a motivational decision support system. clinicaltrials.gov/show/NCT02086162 (first received March 13 2014).
Nichter 2018 {published data only}
-
- Nichter M, Mini GK, Thankappan KR. Low‐level smoking among diabetes patients in India: a smoking cessation challenge. Clinical Epidemiology and Global Health 2018;6(4):176‐80. [DOI: 10.1016/j.cegh.2017.11.005] - DOI
Pardavila‐Belio 2015 {published data only}
Parker 2007 {published data only}
Persson 2006 {published data only}
-
- Persson LG, Hjalmarson A. Smoking cessation in patients with diabetes mellitus: results from a controlled study of an intervention programme in primary healthcare in Sweden. Scandinavian Journal of Primary Health Care 2006;24:75‐80. - PubMed
Pineiro 2014 {published data only}
-
- Pineiro B, Rio Elena F, Lopez‐Duran A, Becona E. Does motivational interviewing improve the effectiveness of a psychological treatment to quit smoking?. Anales de Psicologia 2014;30:124‐33.
Polosa 2011 {published data only}
-
- Polosa R, Caponnetto P, Caruso M. Training pharmacists in the stage‐of‐change model of smoking cessation: a randomised controlled trial in Sicily (abstract). European Respiratory Society Annual Congress; 2011 Sept 24‐28; Amsterdam, the Netherlands. 2011; Vol. 38:780s [P4247].
Reitzel 2010 {published data only}
Rigotti 1997 {published data only}
-
- Rigotti NA, Arnsten JH, McKool KM, Wood‐Reid KM, Pasternak RC, Singer DE. Efficacy of a smoking cessation program for hospital patients. Archives of Internal Medicine 1997;157(22):2653‐60. - PubMed
Rigotti 2006 {published data only}
Rogers 2016 {published data only}
-
- Rogers ES, Smelson DA, Gillespie CC, Elbel B, Poole S, Hagedorn HJ, et al. Telephone smoking‐cessation counseling for smokers in mental health clinics: a patient‐randomized controlled trial. American Journal of Preventive Medicine 2016;50(4):518‐27. [DOI: 10.1016/j.amepre.2015.10.004] - DOI - PubMed
Schuck 2014 {published data only}
-
- Schuck K, Bricker JB, Otten R, Kleinjan M, Brandon TH, Engels RC. Effectiveness of proactive quitline counselling for smoking parents recruited through primary schools: results of a randomized controlled trial. Addiction 2014;109(5):830‐41. [DOI: ] - PubMed
-
- Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Predictors of cessation treatment outcome and treatment moderators among smoking parents receiving quitline counselling or self‐help material.. Preventive Medicine 2014;69:126‐31. [DOI: ] - PubMed
-
- Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Promoting smoking cessation among parents: effects on smoking‐related cognitions and smoking initiation in children. Addictive Behaviors 2015;40:66‐72. [DOI: ] - PubMed
Severson 2009 {published data only}
Sherbot 2005 {published data only}
-
- Sherbot NA. The use of motivational enhancement therapy and the quit 4 life program as a means to facilitate adolescent smoking cessation. Dissertation Abstracts International: Section B: the Sciences and Engineering 2005;66:574.
Sims 2013 {published data only}
Skov‐Ettrup 2016 {published data only}
Smith 2001 {published data only}
-
- Smith SS, Jorenby DE, Fiore MC, Anderson JE, Mielke MM, Beach KE, et al. Strike while the iron is hot: can stepped‐care treatments resurrect relapsing smokers?. Journal of Consulting and Clinical Psychology 2001;69(3):429‐39. - PubMed
Sobell 2017 {published data only}
Steinberg 2016 {published data only}
Stotts 2002 {published data only}
-
- Stotts AL, Diclemente CC, Dolan‐Mullen Patricia. One‐to‐one: a motivational intervention for resistant pregnant smokers. Addictive Behaviors 2002;27(2):275‐92. - PubMed
Stotts 2009 {published data only}
-
- Stotts AL, Groff JY, Velasquez MM, Benjamin‐Garner R, Green C, Carbonari JP, et al. Ultrasound feedback and motivational interviewing targeting smoking cessation in the second and third trimesters of pregnancy. Nicotine & Tobacco Research 2009;11(8):961‐8. [DOI: 10.1093/ntr/ntp095] - DOI - PMC - PubMed
Tappin 2000 {published data only}
-
- Tappin DM, Lumsden MA, McIntyre D, Mckay C, Gilmour WH, Webber R, et al. A pilot study to establish a randomized trial methodology to test the efficacy of a behavioural intervention. Health Education Research 2000;15(4):491‐502. - PubMed
Tappin 2005 {published data only}
-
- Tappin DM, Lumsden MA, Gilmour WH, Crawford F, McIntyre D, Stone DH, et al. Randomised controlled trial of home based motivational interviewing by midwives to help pregnant smokers quit or cut down. BMJ (Clinical Research Edition) 2005;331(7513):373‐7. [DOI: 10.1136/bmj.331.7513.373] - DOI - PMC - PubMed
Thomsen 2010 {published data only}
Van Rossem 2015 {published data only}
-
- Rossem C, Spigt M, Smit ES, Viechtbauer W, Mijnheer KK, Schayck CP, et al. Combining intensive practice nurse counselling or brief general practitioner advice with varenicline for smoking cessation in primary care: study protocol of a pragmatic randomized controlled trial. Contemporary Clinical Trials 2015;41:298‐312. [DOI: 10.1016/j.cct.2015.01.017] - DOI - PubMed
Wakefield 2004 {published data only}
-
- Wakefield M, Olver I, Whitford H, Rosenfeld E. Motivational interviewing as a smoking cessation intervention for patients with cancer: randomized controlled trial. Nursing Research 2004;53(6):396‐405. - PubMed
Weaver 2015 {published data only}
-
- Weaver KE, Kaplan S, Griffin L, Urbanic J, Zbikowski S, Danhauer SC. Satisfaction with a quitline‐based smoking cessation intervention among cancer survivors. Cancer Epidemiology, Biomarkers & Prevention 2015;24(4):759. [DOI: 10.1158/1055-9965.EPI-15-0100] - DOI
References to studies awaiting assessment
Zhou 2014 {published data only}
-
- Zhou Y, Feng X, Yu L, Gu Y. Research on the effects of motivational interviewing in smoking cessation for patients with coronary heart diseases. Cardiology (Switzerland) 2014;129:135.
References to ongoing studies
Lloyd‐Richardson 2003 {published data only}
-
- Lloyd‐Richardson EE, Niaura R, Abrams DB. Informed development of smoking cessation interventions for college students (POS4‐25). Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003 Feb 19‐22; New Orleans, Louisiana. 2003.
NCT01387516 {published data only}
-
- NCT01387516. Motivation and skills for detained teen smokers. ClinicalTrials.gov/show/NCT01387516 (first received 4 July 2011).
NCT02905656 {published data only}
-
- NCT02905656. Strategies to promote cessation in smokers who are not ready to quit. ClinicalTrials.gov/show/NCT02905656 (first received 5 January 2016).
NCT03002883 {published data only}
-
- NCT03002883. STAND community college tobacco cessation trial. ClinicalTrials.gov/show/NCT03002883 (first received 5 January 2016).
Additional references
Britt 2002
-
- Britt E, Hudson SM, Blampied NM. Motivational Interviewing in health settings: a review. Patient Education and Counselling 2004;53(2):147‐55. - PubMed
Burckhardt 2003
Cahill 2010
CDC 2017
-
- Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2000–2015. Morbidity and Mortality Weekly Report 2017;65(52):1457‐64. - PubMed
Chamberlain 2017
Cheng 2015
Cowlishaw 2012
EuroQol Group 1990
-
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. - PubMed
Foxcroft 2016
Gates 2016
GBD 2015 Risk Factors Collaborators 2016
-
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1659‐724. [DOI: 10.1016/S0140-6736(16)31679-8] - DOI - PMC - PubMed
Heckman 2010
Hettema 2010
-
- Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta‐analytic review. Journal of Consulting and Clinical Psychology 2010;78(6):868‐84. [PUBMED: 21114344] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jelsma 2015
-
- Jelsma JGM, Mertens V‐C, Forsberg L, Forsberg L. How to measure motivational interviewing fidelity in randomized controlled trials: practical recommendations. Contemporary Clinical Trials 2015;43:93‐9. [DOI: ] - PubMed
Jha 2011
-
- Jha P. Avoidable deaths from smoking: a global perspective. Public Health Reviews 2011;33(2):569‐600.
Klimas 2018
Lawendowski 1998
-
- Lawendowski LA. A motivational intervention for adolescent smokers. Preventive Medicine 1998;5(3):A39‐46. - PubMed
Markland 2005
-
- Markland D, Ryan RM, Tobin VJ, Rollnick S. Motivational interviewing and self–determination theory. Journal of Social and Clinical Psychology 2005;24(6):811‐31.
Mbuagbaw 2012
Miller 1983
-
- Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy 1983;11:147‐72.
Miller 1994
-
- Miller WR. Motivational interviewing. III. On the ethics of motivational interviewing. Behavioural and Cognitive Psychotherapy 1994;22(2):111‐23.
Miller 2002
-
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. Guilford Press, 2002.
Miller 2009
-
- Miller WR, Rollnick S. Ten things motivational interviewing is not. Behavioural and Cognitive Psychotherapy 2009;37:129‐140. - PubMed
Miller 2013
-
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd Edition. New York: Guildford Press, 2013.
Morton 2015
-
- Morton K, Beauchamp M, Prothero A, Joyce L, Saunders L, Spencer‐Bowdage S, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychology Review 2015;9(2):205‐23. [https://doi.org/10.1080/17437199.2014.882006] - PubMed
Pierson 2007
-
- Pierson HM, Hayes SC, Gifford EV, Roget N, Padilla M, Bissett R, et al. An examination of the motivational interviewing treatment integrity code. Journal of Substance Abuse Treatment 2007;32(1):11‐7. [DOI: ] - PubMed
Rollnick 1992
-
- Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. Journal of Mental Health 1992;1:25‐37.
Rollnick 1995
-
- Rollnick SR, Miller WR. What is motivational interviewing?. Behavioural and Cognitive Psychotherapy 1995;23(4):325‐34. - PubMed
Ryan 2000
-
- Ryan RM, Deci EL. Self‐determination theory and the facilitation of intrinsic motivation, social development, and well‐being. American Psychologist 2000;55(1):68‐78. - PubMed
References to other published versions of this review
Lai 2008
Lai 2010
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

