TNF-α blockers for the treatment of Kawasaki disease in children
- PMID: 31425625
- PMCID: PMC6953355
- DOI: 10.1002/14651858.CD012448.pub2
TNF-α blockers for the treatment of Kawasaki disease in children
Abstract
Background: Kawasaki disease (KD) is an acute inflammatory vasculitis (inflammation of the blood vessels) that mainly affects children between six months and five years of age. The vasculitis primarily impacts medium-sized blood vessels, especially in the coronary arteries. In most children, intravenous immunoglobulin (IVIG) and aspirin therapy rapidly reduce inflammatory markers, fever, and other clinical symptoms. However, approximately 15% to 20% of children receiving the initial IVIG infusion show persistent or recurrent fever and are classified as IVIG-resistant. Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine that plays an important role in host defence against infections and in immune responses. Several studies have established that blocking TNF-α is critical for obtaining anti-inflammatory effects in children with KD, thus, there is a need to identify benefits and risks of TNF-α blockers for the treatment of KD.
Objectives: To evaluate the efficacy and safety of using TNF-α blockers (i.e. infliximab and etanercept) to treat children with Kawasaki disease.
Search methods: The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL databases, the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials register to 19 September 2018. We also undertook reference checking of grey literature.
Selection criteria: We included randomised controlled trials (RCTs) that compared TNF-α blockers (i.e. infliximab and etanercept) to placebo or other drugs (including retreatment with IVIG) in children with KD, reported in abstract or full-text.
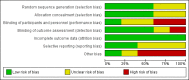
Data collection and analysis: Two review authors independently applied the study selection criteria, assessed risk of bias and extracted data. When necessary, we contacted study authors for additional information. We used GRADE to assess the certainty of the evidence.
Main results: We included five trials from 14 reports, with a total of 494 participants. All included trials were individual RCTs that examined the effect of TNF-α blockers for KD.Five trials (with 494 participants) reported the incidence of treatment resistance. TNF-α blockers reduced the incidence of treatment resistance (TNF-α blocker intervention group 30/237, control group 58/257; risk ratio (RR) 0.57, 95% confidence interval (CI) 0.38 to 0.86; low-certainty evidence).Four trials reported the incidence of coronary artery abnormalities (CAAs). Three trials (with 270 participants) contributed data to the meta-analysis, since we could not get the data needed for the analysis from the fourth trial. There was no clear difference between groups in the incidence of CAAs (TNF-α blocker intervention group 8/125, control group 9/145; RR 1.18, 95% CI 0.45 to 3.12; low-certainty evidence).Three trials with 250 participants reported the adverse effect 'infusion reactions' after treatment initiation. The TNF-α blocker intervention decreased infusion reactions (TNF-α blocker intervention group 0/126, control group 15/124; RR 0.06, 95% CI 0.01 to 0.45; low-certainty evidence).Two trials with 227 participants reported the adverse effect 'infections' after treatment initiation. There was no clear difference between groups (TNF-α blocker intervention group 7/114, control group 10/113; RR 0.68, 95% CI 0.33 to 1.37; low-certainty evidence).One trial (with 31 participants) reported the adverse effect 'cutaneous reactions' (rash and contact dermatitis). There was no clear difference between the groups for incidence of rash (TNF-α blocker intervention group 2/16, control group 0/15; RR 4.71, 95% CI 0.24 to 90.69; very low-certainty evidence) or for incidence of contact dermatitis (TNF-α blocker intervention group 1/16, control group 3/15; RR 0.31, 95% CI 0.04 to 2.68; very low-certainty evidence).No trials reported other adverse effects such as injection site reactions, neutropenia, infections, demyelinating disease, heart failure, malignancy, and induction of autoimmunity.
Authors' conclusions: We found a limited number of RCTs examining the effect of TNF-α blockers for KD. In summary, low-certainty evidence indicates that TNF-α blockers have beneficial effects on treatment resistance and the adverse effect 'infusion reaction' after treatment initiation for KD when compared with no treatment or additional treatment with IVIG. Further research will add to the evidence base. Due to the small number of underpowered trials contributing to the analyses, the results presented should be treated with caution. Further large high quality trials with timing and type of TNF-α blockers used are needed to determine the effects of TNF-α blockers for KD.
Conflict of interest statement
NY: none known
KL: none known
TS: none known
KI: none known
TK: none known
EO: none known
RM: none known
Figures
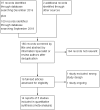






Update of
References
References to studies included in this review
Burns 2008 {published data only}
-
- NCT00271570. Infliximab (Remicade) for patients with acute Kawasaki disease. clinicaltrials.gov/ct2/show/NCT00271570 (first received 2 January 2006).
Mori 2018 {published data only}
-
- Mori M, Hara T, Kikuchi M, Shimizu H, Miyamoto T, Iwashima S, et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open‐label, active‐controlled, parallel‐group, multicenter trial. Supplementary information. Available from https://static‐content.springer.com/esm/art%3A10.1038%2Fs41598‐017‐18387‐7/MediaObjects.... - PMC - PubMed
-
- NCT01596335. Clinical study of TA‐650 in patients with refractory Kawasaki disease. clinicaltrials.gov/ct2/show/NCT01596335 (first received 11 May 2012).
Portman 2018 {published data only}
-
- Portman MA, Dahdah N, Olson AK, Slee A, Choueiter N, Altman C, et al. Multicenter, randomized, double‐blind trial of etanercept in acute Kawasaki disease. Circulation 2018;136:A15447.
-
- Portman MA, Dahdah N, Olson AK, et al. A randomized multicenter double‐blind placebo controlled trial of etanercept acute Kawasaki Disease. Progress and harmony for Kawasaki disease. The 12th International Kawasaki Disease Symposium; 2018 June 12‐15; Pacifico Yokohama, Japan. 2018:MPS2‐11.
-
- Portman MA, Olson A, Soriano B, Dahdah N, Williams R, Kirkpatrick E. Etanercept as adjunctive treatment for acute Kawasaki disease: study design and rationale. American Heart Journal 2011;161(3):494‐9. - PubMed
Tremoulet 2014 {published data only}
-
- Jaggi P, Wang W, Printz B, Burns J, Kovalchin J, Tremoulet A. Fever patterns in KD patients after treatment with IVIG. Circulation 2015;131(Suppl 2):Abstract O.38.
-
- NCT00760435. Infliximab plus intravenous immunoglobulin for the primary treatment of Kawasaki disease. clinicaltrials.gov/ct2/show/NCT00760435 (first received 26 September 2008).
-
- Tremoulet A. TNFα blockade in Kawasaki disease: putting out the fire. Pediatrics International 2012;54 (Suppl 1):53‐4.
-
- Tremoulet AH, Jain S, Jaggi P, Jimenez‐Fernandez S, Pancheri JM, Sun X, et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double‐blind, placebo‐controlled trial. Lancet 2014;383(9930):1731‐8. - PubMed
Youn 2016 {published data only}
-
- Youn Y, Kim J, Hong YM, Sohn S. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin. Pediatric Infectious Disease Journal 2016;35(4):457‐9. - PubMed
-
- Youn Y, Kim J, Hong YM, Sohn S. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin. Supplementary information. Available from http://download.lww.com/wolterskluwer_vitalstream_com/PermaLink/INF/C/IN.... - PubMed
-
- Youn Y, Kim J, Hong YM, Sohn S. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin. Supplementary information. Available from https://download.lww.com/wolterskluwer_vitalstream_com/PermaLink/INF/C/I.... - PubMed
References to studies excluded from this review
ChiCTR‐IOR‐16007862 {published data only}
-
- ChiCTR‐IOR‐16007862. Research on individualized treatment for intravenous immunoglobulin resistant children with Kawasaki disease. chictr.org.cn/showprojen.aspx?proj=13290. ChiCTR‐IOR‐16007862, (first received 30 January 2016).
References to ongoing studies
NCT02298062 {published data only}
-
- NCT02298062. Infliximab for Kawasaki Disease patients resistant to IVIG: a multicentre, prospective, randomised trial. clinicaltrials.gov/ct2/show/NCT02298062. NCT02298062, (first received 21 November 2014).
Additional references
Aeschlimann 2016
-
- Aeschlimann FA, Yeung RS. TNF and IL‐1 targeted treatment in Kawasaki disease. Current Treatment Options in Rheumatology 2016;2(4):283‐95.
Agarwal 2017
Ayusawa 2005
-
- Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Kawasaki Disease Research Committee. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatrics International 2005;47(2):232‐4. - PubMed
Baumer 2006
Burns 1996
-
- Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. Journal of the American College of Cardiology 1996;28(1):253‐7. - PubMed
Burns 1998
-
- Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma‐globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatric Infectious Disease Journal 1998;17(12):1144‐8. - PubMed
Burns 2005
-
- Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. Journal of Pediatrics 2005;146(5):662‐7. [PUBMED: 15870671] - PubMed
Choueiter 2010
Durongpisitkul 1995
-
- Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta‐analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics 1995;96(6):1057‐61. - PubMed
Feng 2015
Fiers 1991
-
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Letters 1991;285(2):199‐212. - PubMed
Furukawa 1988
-
- Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clinical Immunology and Immunopathology 1988;48(2):247‐51. - PubMed
Furusho 1984
-
- Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High‐dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984;2(8411):1055‐8. - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 17 June 2019. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirono 2009
-
- Hirono K, Kemmotsu Y, Wittkowski H, Foell D, Saito K, Ibuki K, et al. Infliximab reduces the cytokine‐mediated inflammation but does not suppress cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatric Research 2009;65(6):696‐701. - PubMed
Huang 2009
-
- Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, Chen PJ, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003‐2006. Pediatrics 2009;123(3):e401‐5. - PubMed
Hui‐Yuen 2006
-
- Hui‐Yuen JS, Duong TT, Yeung RS. TNF‐alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. Journal of Immunology 2006;176(10):6294‐301. - PubMed
Hur 2019
Imagawa 2009
-
- Imagawa T, Miyamae T, Kishi T, Mori M, Yokota S. Effectiveness of tocilizumab for intravenous immunoglobulin‐resistant Kawasaki disease. Progress in Medicine 2009;29:1166.
Kawasaki 1967
-
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967;16(3):178–222. - PubMed
Khor 2011
-
- Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, Wright VJ, et al. Genome‐wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nature Genetics 2011;43(12):1241‐6. - PubMed
Kim 2014
-
- Kim GB, Han JW, Park YW, Song MS, Hong YM, Cha SH, et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009‐2011. Pediatric Infectious Disease Journal 2014;33(1):24‐7. - PubMed
Kobayashi 2013
-
- Kobayashi T, Kobayashi T, Morikawa A, Ikeda K, Seki M, Shimoyama S, et al. Efficacy of intravenous immunoglobulin combined with prednisolone following resistance to initial intravenous immunoglobulin treatment of acute Kawasaki disease. Journal of Pediatrics 2013;163(2):521‐6. - PubMed
Kodama 2005
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leung 1986
-
- Leung DY, Geha RS, Newburger JW, Burns JC, Fiers W, Lapierre LA, et al. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. Journal of Experimental Medicine 1986;164(6):1958‐72. - PMC - PubMed
Makino 2015
Makino 2019
-
- Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015‐2016. Pediatrics International 2019;61(4):397‐403. [PUBMED: 30786118] - PubMed
Masuda 2018
-
- Masuda H, Kobayashi T, Hachiya A, Nakashima Y, Shimizu H, Nozawa T, et al. Infliximab for the treatment of refractory Kawasaki disease: a nationwide survey in Japan. Journal of Pediatrics 2018;195:115‐20. - PubMed
Matsubara 1990
-
- Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon‐gamma in Kawasaki disease involved coronary‐artery lesions. Clinical Immunology and Immunopathology 1990;56(1):29‐36. - PubMed
McCrindle 2017
-
- McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, Treatment, and Long‐Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017;135(17):e927‐e999. - PubMed
Mori 2012
-
- Mori M, Imagawa T, Hara R, Kikuchi M, Hara T, Nozawa T, et al. Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: report of an open‐label case series. Journal of Rheumatology 2012;39(4):864‐7. - PubMed
Nakamura 2008
Nakamura 2010
Nakamura 2012
Newburger 1986
-
- Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. New England Journal of Medicine 1986;315(6):341‐7. - PubMed
Newburger 2004
-
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110(17):2747‐71. - PubMed
Oates‐Whitehead 2003
Ogawa 2013
-
- Ogawa S, Ayusawa M, Ishii M, Ogino H, Saji T, Nishigaki K, et al. Guidlines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS2013). Available from www.j‐circ.or.jp/guideline/pdf/JCS2013_ogawas_d.pdf 2013.
Ogihara 2014
-
- Ogihara Y, Ogata S, Nomoto K, Ebato T, Sato K, Kokubo K, et al. Transcriptional regulation by infliximab therapy in Kawasaki disease patients with immunoglobulin resistance. Pediatric Research 2014;76(3):287‐93. - PubMed
Oharaseki 2014
-
- Oharaseki T, Yokouchi Y, Yamada H, Mamada H, Muto S, Sadamoto K, et al. The role of TNF‐α in a murine model of Kawasaki disease arteritis induced with a Candida albicans cell wall polysaccharide. Modern Rheumatology/the Japan Rheumatism Association 2014;24(1):120‐8. - PubMed
Onouchi 2008
Onouchi 2010
Onouchi 2012
-
- Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, Hamada H, et al. A genome‐wide association study identifies three new risk loci for Kawasaki disease. Nature Genetics 2012;44(5):517‐21. - PubMed
Onouchi 2018
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sauvaget 2012
-
- Sauvaget E, Bonello B, David M, Chabrol B, Dubus JC, Bosdure E. Resistant Kawasaki disease treated with anti‐CD20. Journal of Pediatrics 2012;160(5):875–6. - PubMed
Schünemann 2009
-
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Lösungsbeitrag zur Übertragbarkeit von Studienergebnissen]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2009;103(6):391–400. - PubMed
Shafferman 2014
Singh 2015
-
- Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Archives of Disease in Childhood 2015;100:1084‐8. - PubMed
Singh 2016
-
- Singh S, Sharma D, Suri D, Gupta A, Rawat A, Rohit MK. Infliximab is the new kid on the block in Kawasaki disease: a single‐centre study over 8 years from North India. Clinical and Experimental Rheumatology 2016;34(3 Suppl 97):S134‐8. - PubMed
Song 2010
Terai 1997
-
- Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. Journal of Pediatrics 1997;131(6):888‐93. - PubMed
Tremoulet 2008
Uehara 2012
Weiss 2004
-
- Weiss JE, Eberhard BA, Chowdhury D, Gottlieb BS. Infliximab as a novel therapy for refractory Kawasaki disease. Journal of Rheumatology 2004;31(4):808‐10. - PubMed
Yanagawa 2004
-
- Yanagawa H, Nakamura Y, Yashiro M, Kawasaki T, editor(s). Epidemiology of Kawasaki disease: a 30‐year achievement. Tokyo: Shindan‐to‐Chiryosha, 2004.
Yanagawa 2006
-
- Yanagawa H, Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K. Incidence of Kawasaki disease in Japan: the nationwide surveys of 1999‐2002. Pediatrics International 2006;48(4):356‐61. [PUBMED: 16911079] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

