Sabotage at the Powerhouse? Unraveling the Molecular Target of 2-Isopropylbenzaldehyde Thiosemicarbazone, a Specific Inhibitor of Aflatoxin Biosynthesis and Sclerotia Development in Aspergillus flavus, Using Yeast as a Model System
- PMID: 31426298
- PMCID: PMC6719062
- DOI: 10.3390/molecules24162971
Sabotage at the Powerhouse? Unraveling the Molecular Target of 2-Isopropylbenzaldehyde Thiosemicarbazone, a Specific Inhibitor of Aflatoxin Biosynthesis and Sclerotia Development in Aspergillus flavus, Using Yeast as a Model System
Abstract
Amongst the various approaches to contain aflatoxin contamination of feed and food commodities, the use of inhibitors of fungal growth and/or toxin biosynthesis is showing great promise for the implementation or the replacement of conventional pesticide-based strategies. Several inhibition mechanisms were found taking place at different levels in the biology of the aflatoxin-producing fungal species such as Aspergillus flavus: compounds that influence aflatoxin production may block the biosynthetic pathway through the direct control of genes belonging to the aflatoxin gene cluster, or interfere with one or more of the several steps involved in the aflatoxin metabolism upstream. Recent findings pointed to mitochondrial functionality as one of the potential targets of some aflatoxin inhibitors. Additionally, we have recently reported that the effect of a compound belonging to the class of thiosemicarbazones might be related to the energy generation/carbon flow and redox homeostasis control by the fungal cell. Here, we report our investigation about a putative molecular target of the 3-isopropylbenzaldehyde thiosemicarbazone (mHtcum), using the yeast Saccharomyces cerevisiae as model system, to demonstrate how the compound can actually interfere with the mitochondrial respiratory chain.
Keywords: aflatoxin inhibitor; mitochondrion; respiratory chain; yeast model system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


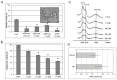
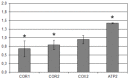
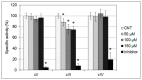

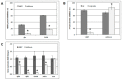
References
-
- Abbas H.K., Wilkinson J., Zablotowicz R., Accinelli C., Abel C., Bruns H., Weaver M. Ecology of Aspergillus flavus, regulation of aflatoxin production, and management strategies to reduce aflatoxin contamination of corn. Toxin Rev. 2009;28:142–153. doi: 10.1080/15569540903081590. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

