The Multiple Roles and Therapeutic Potential of Molecular Chaperones in Prostate Cancer
- PMID: 31426412
- PMCID: PMC6721600
- DOI: 10.3390/cancers11081194
The Multiple Roles and Therapeutic Potential of Molecular Chaperones in Prostate Cancer
Abstract
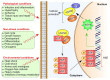
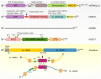
Prostate cancer (PCa) is one of the most common cancer types in men worldwide. Heat shock proteins (HSPs) are molecular chaperones that are widely implicated in the pathogenesis, diagnosis, prognosis, and treatment of many cancers. The role of HSPs in PCa is complex and their expression has been linked to the progression and aggressiveness of the tumor. Prominent chaperones, including HSP90 and HSP70, are involved in the folding and trafficking of critical cancer-related proteins. Other members of HSPs, including HSP27 and HSP60, have been considered as promising biomarkers, similar to prostate-specific membrane antigen (PSMA), for PCa screening in order to evaluate and monitor the progression or recurrence of the disease. Moreover, expression level of chaperones like clusterin has been shown to correlate directly with the prostate tumor grade. Hence, targeting HSPs in PCa has been suggested as a promising strategy for cancer therapy. In the current review, we discuss the functions as well as the role of HSPs in PCa progression and further evaluate the approach of inhibiting HSPs as a cancer treatment strategy.
Keywords: HSPs inhibitors; biomarkers; heat shock proteins (HSPs); molecular chaperones; prostate cancer; therapeutic resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy.Cells. 2019 Dec 24;9(1):60. doi: 10.3390/cells9010060. Cells. 2019. PMID: 31878360 Free PMC article. Review.
-
Heat shock proteins in hepatocellular carcinoma: Molecular mechanism and therapeutic potential.Int J Cancer. 2016 Apr 15;138(8):1824-34. doi: 10.1002/ijc.29723. Epub 2015 Aug 19. Int J Cancer. 2016. PMID: 26853533 Review.
-
Heat Shock Proteins in Cancer Immunotherapy.J Oncol. 2019 Dec 11;2019:3267207. doi: 10.1155/2019/3267207. eCollection 2019. J Oncol. 2019. PMID: 31885572 Free PMC article. Review.
-
Molecular Chaperones in Osteosarcoma: Diagnosis and Therapeutic Issues.Cells. 2021 Mar 30;10(4):754. doi: 10.3390/cells10040754. Cells. 2021. PMID: 33808130 Free PMC article. Review.
-
Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer.Curr Drug Targets. 2018;19(13):1478-1490. doi: 10.2174/1389450118666170823121248. Curr Drug Targets. 2018. PMID: 28831912 Review.
Cited by
-
Overexpression of HSP10 correlates with HSP60 and Mcl-1 levels and predicts poor prognosis in non-small cell lung cancer patients.Cancer Biomark. 2021;30(1):85-94. doi: 10.3233/CBM-200410. Cancer Biomark. 2021. PMID: 32986659 Free PMC article.
-
The chaperone system in cancer therapies: Hsp90.J Mol Histol. 2023 Apr;54(2):105-118. doi: 10.1007/s10735-023-10119-8. Epub 2023 Mar 18. J Mol Histol. 2023. PMID: 36933095 Free PMC article. Review.
-
Crafting a Personalized Prognostic Model for Malignant Prostate Cancer Patients Using Risk Gene Signatures Discovered through TCGA-PRAD Mining, Machine Learning, and Single-Cell RNA-Sequencing.Diagnostics (Basel). 2023 Jun 7;13(12):1997. doi: 10.3390/diagnostics13121997. Diagnostics (Basel). 2023. PMID: 37370891 Free PMC article.
-
Heat Shock Protein 60 in Hepatocellular Carcinoma: Insights and Perspectives.Front Mol Biosci. 2020 Apr 15;7:60. doi: 10.3389/fmolb.2020.00060. eCollection 2020. Front Mol Biosci. 2020. PMID: 32351972 Free PMC article. Review.
-
Priming with HDAC Inhibitors Sensitizes Ovarian Cancer Cells to Treatment with Cisplatin and HSP90 Inhibitors.Int J Mol Sci. 2020 Nov 5;21(21):8300. doi: 10.3390/ijms21218300. Int J Mol Sci. 2020. PMID: 33167494 Free PMC article.
References
-
- Castelletti D., Alfalah M., Heine M., Hein Z., Schmitte R., Fracasso G., Colombatti M., Naim H.Y. Different glycoforms of prostate-specific membrane antigen are intracellularly transported through their association with distinct detergent-resistant membranes. Biochem. J. 2008;409:149–157. doi: 10.1042/BJ20070396. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

