Interplay between Intrinsic and Innate Immunity during HIV Infection
- PMID: 31426525
- PMCID: PMC6721663
- DOI: 10.3390/cells8080922
Interplay between Intrinsic and Innate Immunity during HIV Infection
Abstract
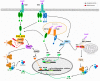
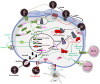
Restriction factors are antiviral components of intrinsic immunity which constitute a first line of defense by blocking different steps of the human immunodeficiency virus (HIV) replication cycle. In immune cells, HIV infection is also sensed by several pattern recognition receptors (PRRs), leading to type I interferon (IFN-I) and inflammatory cytokines production that upregulate antiviral interferon-stimulated genes (ISGs). Several studies suggest a link between these two types of immunity. Indeed, restriction factors, that are generally interferon-inducible, are able to modulate immune responses. This review highlights recent knowledge of the interplay between restriction factors and immunity inducing antiviral defenses. Counteraction of this intrinsic and innate immunity by HIV viral proteins will also be discussed.
Keywords: HIV-1; host restriction factors; immune responses; innate immune sensing; interferon; viral counteraction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Ségéral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

