Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review
- PMID: 31426613
- PMCID: PMC6720215
- DOI: 10.3390/ma12162629
Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review
Abstract
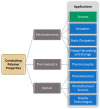
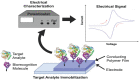
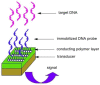
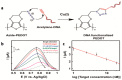
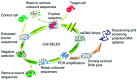
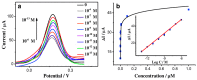
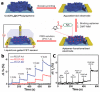
Conducting polymers are of interest due to their unique behavior on exposure to electric fields, which has led to their use in flexible electronics, sensors, and biomaterials. The unique electroactive properties of conducting polymers allow them to be used to prepare biosensors that enable real time, point of care (POC) testing. Potential advantages of these devices include their low cost and low detection limit, ultimately resulting in increased access to treatment. This article presents a review of the characteristics of conducting polymer-based biosensors and the recent advances in their application in the recognition of disease biomarkers.
Keywords: biosensor; conducting polymer; electroactive polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Huang W.S., Humphrey B.D., MacDiarmid A.G. Polyaniline, a Novel Conducting Polymer. Morphology and Chemistry of Its Oxidation and Reduction in Aqueous Electrolytes. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases. 1986;82:2385–2400. doi: 10.1039/f19868202385. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

