Impact of Circadian Disruption on Cardiovascular Function and Disease
- PMID: 31427142
- PMCID: PMC6779516
- DOI: 10.1016/j.tem.2019.07.008
Impact of Circadian Disruption on Cardiovascular Function and Disease
Abstract
The circadian system, that is ubiquitous across species, generates ∼24 h rhythms in virtually all biological processes, and allows them to anticipate and adapt to the 24 h day/night cycle, thus ensuring optimal physiological function. Epidemiological studies show time-of-day variations in adverse cardiovascular (CV) events, and controlled laboratory studies demonstrate a circadian influence on key markers of CV function and risk. Furthermore, circadian misalignment, that is typically experienced by shift workers as well as by individuals who experience late eating, (social) jet lag, or circadian rhythm sleep-wake disturbances, increases CV risk factors. Therefore, understanding the mechanisms by which the circadian system regulates CV function, and which of these are affected by circadian disruption, may help to develop intervention strategies to mitigate CV risk.
Keywords: cardiovascular risk; circadian misalignment; circadian rhythms; shiftwork.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
F.A.J.L.S. has received lecture fees from Bayer HealthCare, Sentara HealthCare, Philips, Vanda Pharmaceuticals, and Pfizer Pharmaceuticals. S.L.C, N.V and J.S.W report no conflicts of interest.
Figures
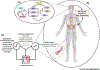


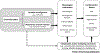

References
-
- Muller JE et al. (1985) Circadian variation in the frequency of onset of acute myocardial infarction. The New England journal of medicine 313 (21), 1315–22. - PubMed
-
- Suarez-Barrientos A et al. (2011) Circadian variations of infarct size in acute myocardial infarction. Heart 97 (12), 970–6. - PubMed
-
- Krantz DS et al. (1996) Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation 93 (7), 1364–71. - PubMed
-
- Organization WH (2011) Global status report on noncommunicable diseases 2010. Geneva: World Health Organization.
Publication types
MeSH terms
Grants and funding
- R01 DK099512/DK/NIDDK NIH HHS/United States
- R01 HL118601/HL/NHLBI NIH HHS/United States
- R01 HL141406/HL/NHLBI NIH HHS/United States
- R01 HL144779/HL/NHLBI NIH HHS/United States
- R01 DK102696/DK/NIDDK NIH HHS/United States
- P01 AG009975/AG/NIA NIH HHS/United States
- R01 HL140574/HL/NHLBI NIH HHS/United States
- R01 HL094806/HL/NHLBI NIH HHS/United States
- R01 DK105072/DK/NIDDK NIH HHS/United States
- R56 HL114765/HL/NHLBI NIH HHS/United States
- R01 HL127146/HL/NHLBI NIH HHS/United States
- K24 HL103845/HL/NHLBI NIH HHS/United States
- R01 HL136567/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

