SUGAR-DIP trial: oral medication strategy versus insulin for diabetes in pregnancy, study protocol for a multicentre, open-label, non-inferiority, randomised controlled trial
- PMID: 31427334
- PMCID: PMC6701578
- DOI: 10.1136/bmjopen-2019-029808
SUGAR-DIP trial: oral medication strategy versus insulin for diabetes in pregnancy, study protocol for a multicentre, open-label, non-inferiority, randomised controlled trial
Abstract
Introduction: In women with gestational diabetes mellitus (GDM) requiring pharmacotherapy, insulin was the established first-line treatment. More recently, oral glucose lowering drugs (OGLDs) have gained popularity as a patient-friendly, less expensive and safe alternative. Monotherapy with metformin or glibenclamide (glyburide) is incorporated in several international guidelines. In women who do not reach sufficient glucose control with OGLD monotherapy, usually insulin is added, either with or without continuation of OGLDs. No reliable data from clinical trials, however, are available on the effectiveness of a treatment strategy using all three agents, metformin, glibenclamide and insulin, in a stepwise approach, compared with insulin-only therapy for improving pregnancy outcomes. In this trial, we aim to assess the clinical effectiveness, cost-effectiveness and patient experience of a stepwise combined OGLD treatment protocol, compared with conventional insulin-based therapy for GDM.
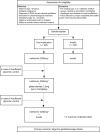
Methods: The SUGAR-DIP trial is an open-label, multicentre randomised controlled non-inferiority trial. Participants are women with GDM who do not reach target glycaemic control with modification of diet, between 16 and 34 weeks of gestation. Participants will be randomised to either treatment with OGLDs, starting with metformin and supplemented as needed with glibenclamide, or randomised to treatment with insulin. In women who do not reach target glycaemic control with combined metformin and glibenclamide, glibenclamide will be substituted with insulin, while continuing metformin. The primary outcome will be the incidence of large-for-gestational-age infants (birth weight >90th percentile). Secondary outcome measures are maternal diabetes-related endpoints, obstetric complications, neonatal complications and cost-effectiveness analysis. Outcomes will be analysed according to the intention-to-treat principle.
Ethics and dissemination: The study protocol was approved by the Ethics Committee of the Utrecht University Medical Centre. Approval by the boards of management for all participating hospitals will be obtained. Trial results will be submitted for publication in peer-reviewed journals.
Trial registration number: NTR6134; Pre-results.
Keywords: clinical trials; diabetes in pregnancy; maternal medicine.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JHD sits on advisory boards for Novo Nordisk A/S. BWM is supported by a National Health and Medical Research Council Practitioner Fellowship (GNT1082548). BWM reports consultancy for ObsEva, Merck KGaA and Guerbet.
Figures
References
-
- Wendland EM, Torloni MR, Falavigna M, et al. . Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012;12:23 10.1186/1471-2393-12-23 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical