Vascular Endothelial Receptor Tyrosine Phosphatase: Identification of Novel Substrates Related to Junctions and a Ternary Complex with EPHB4 and TIE2
- PMID: 31427368
- PMCID: PMC6773558
- DOI: 10.1074/mcp.RA119.001716
Vascular Endothelial Receptor Tyrosine Phosphatase: Identification of Novel Substrates Related to Junctions and a Ternary Complex with EPHB4 and TIE2
Abstract
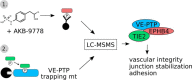
Vascular endothelial protein tyrosine phosphatase (VE-PTP, PTPRB) is a receptor type phosphatase that is crucial for the regulation of endothelial junctions and blood vessel development. We and others have shown recently that VE-PTP regulates vascular integrity by dephosphorylating substrates that are key players in endothelial junction stability, such as the angiopoietin receptor TIE2, the endothelial adherens junction protein VE-cadherin and the vascular endothelial growth factor receptor VEGFR2. Here, we have systematically searched for novel substrates of VE-PTP in endothelial cells by utilizing two approaches. First, we studied changes in the endothelial phosphoproteome on exposing cells to a highly VE-PTP-specific phosphatase inhibitor followed by affinity isolation and mass-spectrometric analysis of phosphorylated proteins by phosphotyrosine-specific antibodies. Second, we used a substrate trapping mutant of VE-PTP to pull down phosphorylated substrates in combination with SILAC-based quantitative mass spectrometry measurements. We identified a set of substrate candidates of VE-PTP, of which a remarkably large fraction (29%) is related to cell junctions. Several of those were found in both screens and displayed very high connectivity in predicted functional interaction networks. The receptor protein tyrosine kinase EPHB4 was the most prominently phosphorylated protein on VE-PTP inhibition among those VE-PTP targets that were identified by both proteomic approaches. Further analysis revealed that EPHB4 forms a ternary complex with VE-PTP and TIE2 in endothelial cells. VE-PTP controls the phosphorylation of each of these two tyrosine kinase receptors. Despite their simultaneous presence in a ternary complex, stimulating each of the receptors with their own specific ligand did not cross-activate the respective partner receptor. Our systematic approach has led to the identification of novel substrates of VE-PTP, of which many are relevant for the control of cellular junctions further promoting the importance of VE-PTP as a key player of junctional signaling.
Keywords: cell adhesion; cell-cell interactions; immunoaffinity; phosphorylation; protein complex analysis; protein phosphatases; substrate identification; substrate trapping; tyrosine kinases.
© 2019 Drexler et al.
Figures





References
-
- Tonks N. K. (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7, 833–846 - PubMed
-
- Yao Z., Darowski K., St-Denis N., Wong V., Offensperger F., Villedieu A., Amin S., Malty R., Aoki H., Guo H., Xu Y., Iorio C., Kotlyar M., Emili A., Jurisica I., Neel B. G., Babu M., Gingras A. C., and Stagljar I. (2017) A Global Analysis of the Receptor Tyrosine Kinase-Protein Phosphatase Interactome. Mol. Cell. 65, 347–360 - PMC - PubMed
-
- Fachinger G., Deutsch U., and Risau W. (1999) Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene 18, 5948–5953 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

