Abscisic acid induced a negative geotropic response in dark-incubated Chlamydomonas reinhardtii
- PMID: 31427663
- PMCID: PMC6700132
- DOI: 10.1038/s41598-019-48632-0
Abscisic acid induced a negative geotropic response in dark-incubated Chlamydomonas reinhardtii
Abstract
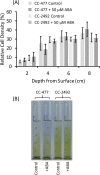
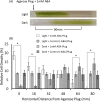
The phytohormone abscisic acid (ABA) plays a role in stresses that alter plant water status and may also regulate root gravitropism and hydrotropism. ABA also exists in the aquatic algal progenitors of land plants, but other than its involvement in stress responses, its physiological role in these microorganisms remains elusive. We show that exogenous ABA significantly altered the HCO3- uptake of Chamydomonas reinhardtii in a light-intensity-dependent manner. In high light ABA enhanced HCO3- uptake, while under low light uptake was diminished. In the dark, ABA induced a negative geotropic movement of the algae to an extent dependent on the time of sampling during the light/dark cycle. The algae also showed a differential, light-dependent directional taxis response to a fixed ABA source, moving horizontally towards the source in the light and away in the dark. We conclude that light and ABA signal competitively in order for algae to position themselves in the water column to minimise photo-oxidative stress and optimise photosynthetic efficiency. We suggest that the development of this response mechanism in motile algae may have been an important step in the evolution of terrestrial plants and that its retention therein strongly implicates ABA in the regulation of their relevant tropisms.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Leliaert F, et al. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012;31:1–46. doi: 10.1080/07352689.2011.615705. - DOI
-
- Hegemann, P. & Berthold, P. Sensory photoreceptors and light control of flagellar activity in The Chlamydomonas Sourcebook: Cell Motility and Behavior, Vol. 3 (ed. Whitman, G.) 395–429 (Acad. Press, Elsevier, London, UK, 2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

