The p21 dependent G2 arrest of the cell cycle in epithelial tubular cells links to the early stage of renal fibrosis
- PMID: 31427681
- PMCID: PMC6700145
- DOI: 10.1038/s41598-019-48557-8
The p21 dependent G2 arrest of the cell cycle in epithelial tubular cells links to the early stage of renal fibrosis
Abstract
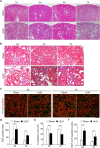
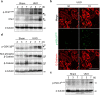
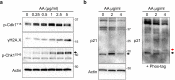
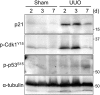
Renal fibrosis is accompanied by the progression of chronic kidney disease. Despite a number of past and ongoing studies, our understanding of the underlying mechanisms remains elusive. Here we explored the progression of renal fibrosis using a mouse model of unilateral ureter obstruction. We found that in the initial stage of damage, where extracellular matrix was not yet deposited, proximal tubular cells arrested at G2 of the cell cycle. Further analyses indicated that the cyclin-dependent kinase inhibitor p21 is partially involved in the G2 arrest after the damage. A newly produced monoclonal antibody against p21 revealed that levels of p21 were sharply upregulated in response to the damage during the initial stage but dropped toward the later stage. To investigate the requirement of p21 for the progression of renal fibrosis, we constructed the novel p21 deficient mice by i-GONAD method. Compared with wild-type mice, p21 deficient mice showed exacerbation of the fibrosis. Thus we propose that during the initial stage of the renal damage, tubular cells arrest in G2 partially depending on p21, thereby safeguarding kidney functions.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

