Prognostic significance of early changes in serum biomarker levels in patients with newly diagnosed metastatic prostate cancer
- PMID: 31427687
- PMCID: PMC6700107
- DOI: 10.1038/s41598-019-48600-8
Prognostic significance of early changes in serum biomarker levels in patients with newly diagnosed metastatic prostate cancer
Abstract
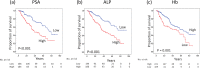
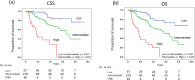
We evaluated the impact of early changes in serum biomarker levels on the survival of patients with metastatic hormone-sensitive prostate cancer (mHSPC) who were initially treated with androgen deprivation therapy (ADT). We retrospectively investigated 330 patients with mHSPC whose serum maker levels were at baseline and at 2-4 months. An optimal Cox regression model was established with the highest optimism-corrected concordance index based on 10-fold cross-validation. The median cancer-specific survival (CSS) and overall survival (OS) were 7.08 and 6.47 years (median follow-up, 2.53 years), respectively. In the final optimal Cox model with serum biomarker levels treated as time-varying covariates, prostate-specific antigen (PSA), hemoglobin (Hb), and alkaline phosphatase (ALP) significantly increased the risk of poor survival in the context of both CSS and OS. Kaplan-Meier curves stratified by the three risk factors of high PSA, low Hb and high ALP desmondtated that median OS were not reached with none of these factors, 6.47 years with one or two factors, and 1.76 years with all three factors.Early changes in serum biomarker levels after ADT may be good prognostic markers for the survival of patients with mHSPC.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Vale CL, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. The Lancet. Oncology. 2016;17:243–256. doi: 10.1016/s1470-2045(15)00489-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

