N-Substituted Pyrrole Derivative 12m Inhibits HIV-1 Entry by Targeting Gp41 of HIV-1 Envelope Glycoprotein
- PMID: 31427969
- PMCID: PMC6688628
- DOI: 10.3389/fphar.2019.00859
N-Substituted Pyrrole Derivative 12m Inhibits HIV-1 Entry by Targeting Gp41 of HIV-1 Envelope Glycoprotein
Abstract
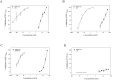
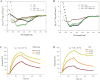
The combination of three or more antiviral agents that act on different targets is known as highly active antiretroviral therapy (HAART), which is widely used to control HIV infection. However, because drug resistance and adverse effects occur after long-term administration, an increasing number of HIV/AIDS patients do not tolerate HAART. It is necessary to continue developing novel anti-HIV drugs, particularly HIV entry/fusion inhibitors. Our group previously identified a small-molecule compound, NB-64, with weak anti-HIV activity. Here, we found that N-substituted pyrrole derivative 12m (NSPD-12m), which was derived from NB-64, had strong anti-HIV-1 activity, and NSPD-12m-treated cells showed good viability. The mechanism of action of NSPD-12m might be targeting the gp41 transmembrane subunit of the HIV envelope glycoprotein, thus inhibiting HIV entry. Site-directed mutagenesis confirmed that a positively charged lysine residue (K574) located in the gp41 pocket region is pivotal for the binding of NSPD-12m to gp41. These findings suggest that NSPD-12m can serve as a lead compound to develop novel virus entry inhibitors.
Keywords: HIV-1 entry inhibitor; cell–cell fusion; gp41 envelope; six-helix bundle; small molecular compound.
Figures




References
LinkOut - more resources
Full Text Sources

