Identification of Two Missense Mutations in DUOX1 (p.R1307Q) and DUOXA1 (p.R56W) That Can Cause Congenital Hypothyroidism Through Impairing H2O2 Generation
- PMID: 31428054
- PMCID: PMC6688124
- DOI: 10.3389/fendo.2019.00526
Identification of Two Missense Mutations in DUOX1 (p.R1307Q) and DUOXA1 (p.R56W) That Can Cause Congenital Hypothyroidism Through Impairing H2O2 Generation
Abstract
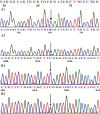
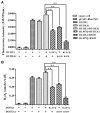
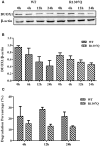
Context: The DUOX/DUOXA systems play a key role in H2O2 generation in thyroid cells, which is required for iodine organification and thyroid hormone synthesis. DUOX2/DUOXA2 defects can cause congenital hypothyroidism (CH), but it is unknown whether DUOX1/DUOXA1 mutations can also cause CH. Objective: We aimed to identify DUOX1/DUOXA1 mutations and explore their role in the development of CH by investigating their functional impacts on H2O2 generation. Patients and Methods: Forty-three children with CH with goiter were enrolled, in whom all exons and flanking intronic regions of DUOX1/DUOXA1 were directly sequenced. We characterized the functional effects of identified mutations on the expression of DUOX1 and DUOXA1 and H2O2 generation. Results: We identified a heterozygous DUOX1 missense mutation (G > A base substitution at nucleotide 3920 in exon 31) that changed a highly conserved arginine to glutamine at residual 1307 (p.R1307Q) in patient 1. A heterozygous-missense mutation (c.166 C>T; p.R56W) was identified in DUOXA1 in patient 2. Functional studies demonstrated that both p.R1307Q mutant or p.R56W mutant decreased the DUOX1 expression at mRNA and protein levels, with a corresponding impairment in H2O2 generation (P < 0.01). The results also showed that intact DUOXA1 was required for full activity of DUOX1 and H2O2 generation. Conclusions: We have identified two heterozygous missense mutations in DUOX1 and DUOXA1 in two patients that can cause CH through disrupting the coordination of DUOX1 and DUOXA1 in the generation of H2O2. This study for the first time demonstrates that the DUOX1/DUOXA1 system, if genetically defective, can cause CH.
Keywords: DUOX1; DUOXA1; H2O2 generation; congenital hypothyroidism; mutation.
Figures



References
LinkOut - more resources
Full Text Sources

